��Ŀ����
 ijͬѧ������ͼ��ʾ������ʵ�飺����ʯ���ܽ���ˮ���ȣ���
ijͬѧ������ͼ��ʾ������ʵ�飺����ʯ���ܽ���ˮ���ȣ�����1��ʵ��1�г���ʯ��ˮ��������
ʯ��ˮ�����
ʯ��ˮ�����
����������Ӧ �Ļ�ѧ����ʽ��CaCO3+2HCl�TCaCl2+CO2��+H2O
CaCO3+2HCl�TCaCl2+CO2��+H2O
��Ca��OH��2+CO2�TCaCO3��+H2O
Ca��OH��2+CO2�TCaCO3��+H2O
��ʵ��2�г���ʯ��ˮ��������ʯ��ˮ�����
ʯ��ˮ�����
�������������ԭ������ʯ����ˮ��Ӧ��������������ʹ��Һ���¶����ߣ�����ʯ�ҵ��ܽ�����¶ȵ����߶���С�������������ƻ����ܽ�ȼ�С������
��ʯ����ˮ��Ӧ��������������ʹ��Һ���¶����ߣ�����ʯ�ҵ��ܽ�����¶ȵ����߶���С�������������ƻ����ܽ�ȼ�С������
����������1���Ӷ�����̼��ʹ�����ʯ��ˮ����ǵĽǶȽ��з�����
��2�����������Ƶ��ܽ�����¶ȵı仯���仯�ĽǶȽ��з�����
��2�����������Ƶ��ܽ�����¶ȵı仯���仯�ĽǶȽ��з�����
����⣺��1��̼��������ᷴӦ�����ɶ�����̼��������̼��ʹ�����ʯ��ˮ����ǣ���Ӧ����ʽΪCaCO3+2HCl�TCaCl2+CO2��+H2O��Ca��OH��2+CO2�TCaCO3��+H2O��
��2�������ƺ�ˮ��Ӧ�ų��������ȣ�ʹװ���ڵ��¶����ߣ����������Ƶ��ܽ�����¶ȵ����߶���С������ɲ����������ƽᾧ���������Գ����ʯ��ˮ����ǣ�
�ʴ�Ϊ����1��ʯ��ˮ����ǣ�CaCO3+2HCl�TCaCl2+CO2��+H2O��Ca��OH��2+CO2�TCaCO3��+H2O��ʯ��ʯ�����ᷴӦ�����Ķ�����̼ʹʯ��ˮ����ǣ�
��2��ʯ��ˮ����ǣ���ʯ����ˮ��Ӧ��������������ʹ��Һ���¶����ߣ�����ʯ�ҵ��ܽ�����¶ȵ����߶���С�������������ƻ����ܽ�ȼ�С��������
��2�������ƺ�ˮ��Ӧ�ų��������ȣ�ʹװ���ڵ��¶����ߣ����������Ƶ��ܽ�����¶ȵ����߶���С������ɲ����������ƽᾧ���������Գ����ʯ��ˮ����ǣ�
�ʴ�Ϊ����1��ʯ��ˮ����ǣ�CaCO3+2HCl�TCaCl2+CO2��+H2O��Ca��OH��2+CO2�TCaCO3��+H2O��ʯ��ʯ�����ᷴӦ�����Ķ�����̼ʹʯ��ˮ����ǣ�
��2��ʯ��ˮ����ǣ���ʯ����ˮ��Ӧ��������������ʹ��Һ���¶����ߣ�����ʯ�ҵ��ܽ�����¶ȵ����߶���С�������������ƻ����ܽ�ȼ�С��������
������������Ҫ���������ʯ��ˮ����ǵ����ַ�����Ҫ��ѧ���ڷ�������ʱһ��Ҫ����ȫ�棬��Ҫһ����ʯ��ˮ����Ǿ���Ϊ��ͨ���˶�����̼��

��ϰ��ϵ�д�
�����Ŀ
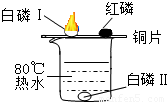
Ϊ̽������ȼ�յ�������ijͬѧ������ͼ��ʾ��ʵ�飮ʵ�鷢��ˮ�еİ���ͭƬ�ϵĺ���ûȼ�գ�ͭƬ�ϵİ����Ż�ȼ�գ���֪�����Ż��40�棬�����Ż��240�森���Դ�ʵ�����ʶ������ǣ�������
| �� | A�� | ��ȼ��Ҫ�������Ӵ� |
| �� | B�� | ����ȼ���¶ȱ���Ҫ�ﵽ�Ż�� |
| �� | C�� | ͭƬ�ϵĺ���ûȼ�գ���Ϊ���ײ��ǿ�ȼ�� |
| �� | D�� | �ձ�����ˮ�����üȹ�����ʹ���ע����������� |
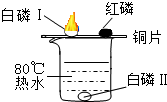 ��2013?������Ϊ̽������ȼ�յ�������ijͬѧ������ͼ��ʾ��ʵ�飮ʵ�鷢��ˮ�еİ���ͭƬ�ϵĺ���ûȼ�գ�ͭƬ�ϵİ����Ż�ȼ�գ���֪�����Ż��40�棬�����Ż��240�森���Դ�ʵ�����ʶ������ǣ�������
��2013?������Ϊ̽������ȼ�յ�������ijͬѧ������ͼ��ʾ��ʵ�飮ʵ�鷢��ˮ�еİ���ͭƬ�ϵĺ���ûȼ�գ�ͭƬ�ϵİ����Ż�ȼ�գ���֪�����Ż��40�棬�����Ż��240�森���Դ�ʵ�����ʶ������ǣ�������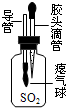 ��2013?����ģ�⣩��ǰ��һ���Կ��ӡ�һ���Է��еİ�ȫ�����ܵ�����ע��ר�ҽ���˵�������һ���Կ������õ�ԭ�϶����ʵؽϺõ�ľ�ģ���������ӹ������Ǻܶ�С����Ϊ�˽��ͳɱ���ʹ������ľ�ģ�Ϊ��ʹ���ӿ���ȥ���ס����£����dz������Ѭ��Ư�ף�������SO2���س��꣬ͬʱ����к���Ǧ�������ؽ�����Ҳ���������Ǧ�ж����ж���
��2013?����ģ�⣩��ǰ��һ���Կ��ӡ�һ���Է��еİ�ȫ�����ܵ�����ע��ר�ҽ���˵�������һ���Կ������õ�ԭ�϶����ʵؽϺõ�ľ�ģ���������ӹ������Ǻܶ�С����Ϊ�˽��ͳɱ���ʹ������ľ�ģ�Ϊ��ʹ���ӿ���ȥ���ס����£����dz������Ѭ��Ư�ף�������SO2���س��꣬ͬʱ����к���Ǧ�������ؽ�����Ҳ���������Ǧ�ж����ж���