��Ŀ����
����Ŀ����9�֣���ѧ���������������
��1������������Ʒ�����л��ϳ������������ɵ��� ������ţ���

A���մɱ� B��������� C�������� D���������
��2�����������������������ڣ���Ҫ��ȡ�϶�ĵ����ʡ�����ʳ���е����ʺ�����ߵ���
������ţ���
A����B��������C������ D������
��3������ˮƿ��ʱ����ˮ���Զ����������˵��������ˮ�е��ܽ���� �й���������ˮ�Ժ�������á���˵��������ܽ�Ȼ��� �й���
��4����ϴ�Ӽ���ϴ���ۣ���������ϴ�Ӽ��� ���á�
��5������3��22���ǵڶ�ʮ����������ˮ������3��22-28���ǵڶ�ʮ�߽����й�ˮ���������Ϲ�ȷ��2014��������ˮ������������������ˮ����Դ��(Water and Energy) ��ˮ�������������������������
��ͼ��ʾij������ˮ�ľ������̣���ش��������⣺
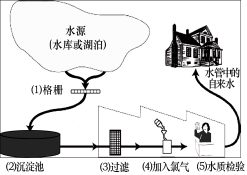
����Ȼˮ�к����������ʣ����������������������˺�����ȷ������������о����̶���ߵķ����� ��
��Ӳˮ����������������ܶ��鷳�������п��� ������Ӳˮ����ˮ������ �ķ���������ˮ��Ӳ�ȡ�
��ClO2����һ������ˮ����������������������Cl2��������ˮ����������ȡClO2�ķ�Ӧ����ʾ��ͼ���£�
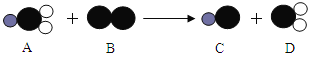
�����У�![]() ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�![]() ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�![]() ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�
�÷�Ӧ�Ļ�ѧ����ʽ�� ��
���𰸡���1��C ��2��A ��3��ѹǿ �¶� ��4���黯
��5�������� ������ˮ ��� ��2NaClO2+Cl2==2ClO2+2NaCl
��������
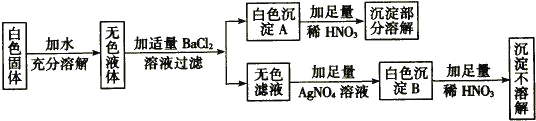
���������(1) A���մɱ��������ǽ������ϣ�B������������ڽ������ϣ�C�������������л��ϳ�������D���������Ҳ�������ǽ������ϣ���ѡC
(2) ���A�⡢Ƥ����ë�����㡢�ǵ���Ҫ�ɷ֡����ࡢ���ࡢ���ࡢţ�⡢���⣬ֲ������ӣ��绨�������ȶ����зḻ�ĵ����ʣ���ѡA
(3)�����ܽ�����¶ȵ����߶����ͣ���ѹǿ���������������ˮƿ��ʱ����ˮ���Զ����������˵��������ˮ�е��ܽ����ѹǿ�йأ�������ˮ�Ժ�������á���˵��������ܽ�Ȼ����¶��й�
��4����ϴ�Ӽ���ϴ���ۣ���������ϴ�Ӽ����黯����
��5���������̶���ߵķ�����������Ӳˮ����ˮ�������÷���ˮ���и�����������ĭ���ٵ���Ӳˮ����ĭ�϶������ˮ�������г�������ķ���������ˮ��Ӳ��
���������ṹʾ��ͼ������A�Ļ�ѧʽΪNaClO2��B�Ļ�ѧʽΪCl2��C�Ļ�ѧʽΪNaCl,D�Ļ�ѧʽΪClO2���÷�Ӧ�Ļ�ѧ����ʽ����2NaClO2+Cl2==2ClO2+2NaCl