��Ŀ����
��6�֣����������־��һ�����һ���ҵ��չ��ˮƽ����ҵ�����������Ҫ����Ϊ��
�����ջ�����4FeS2+11O2 2Fe2O3 + 8SO2
2Fe2O3 + 8SO2
��SO2ת��ΪSO3 ����ŨH2SO4����SO3 ��ش�������⣺
��1������������̢����γɵĹ���ʣ��� ��
��2��SO2������CO2���ƵĻ�ѧ���ʡ������������ʯ������β���е�SO2����д���˷�Ӧ�Ļ�ѧ����ʽ ��
��3���ڢڲ���Ӧ��һ�����Ϸ�Ӧ�����ж�ʵ�ִ˷�Ӧ����һ��Ӧ���� ��
��4����SO2��Cl2ͨ��ˮ����ǡ����ȫ��Ӧ���������ֳ������ᣬ��������л�ѧ����ʽ��Cl2+SO2+2H2O=== +2HCl
��5��ʵ����ϡ��Ũ����ķ����ǣ�
��
�����ջ�����4FeS2+11O2
 2Fe2O3 + 8SO2
2Fe2O3 + 8SO2��SO2ת��ΪSO3 ����ŨH2SO4����SO3 ��ش�������⣺
��1������������̢����γɵĹ���ʣ��� ��
��2��SO2������CO2���ƵĻ�ѧ���ʡ������������ʯ������β���е�SO2����д���˷�Ӧ�Ļ�ѧ����ʽ ��
��3���ڢڲ���Ӧ��һ�����Ϸ�Ӧ�����ж�ʵ�ִ˷�Ӧ����һ��Ӧ���� ��
��4����SO2��Cl2ͨ��ˮ����ǡ����ȫ��Ӧ���������ֳ������ᣬ��������л�ѧ����ʽ��Cl2+SO2+2H2O=== +2HCl
��5��ʵ����ϡ��Ũ����ķ����ǣ�
��
��1����Ϊ����ԭ�ϣ�1�֣�
��2��SO2+Ca(OH)2=== CaSO3��+H2O��2�֣��������ûд�û�ѧ����ʽ�еġ�������ȥ�֣� ��3��O2��1�֣� ��4��H2SO4��1�֣�
��5����Ũ��������������ע��ˮ������Ͻ��衣��1�֣�
��2��SO2+Ca(OH)2=== CaSO3��+H2O��2�֣��������ûд�û�ѧ����ʽ�еġ�������ȥ�֣� ��3��O2��1�֣� ��4��H2SO4��1�֣�
��5����Ũ��������������ע��ˮ������Ͻ��衣��1�֣�
���ջ�����ʱ���ɵĹ���������������������¯������������������������������������SO2��C02һ������������������Է��ն�����̼��ʯ��ˮ��Ӧ�ķ���ʽ������д����������ʯ��ˮ��Ӧ�ķ���ʽ����3�����������������Ӧ��������������4����������ͨ��������ˮ��Һ�з�Ӧ������������ᣬ��5������Ũ�����ϡ�Ͳ�����ע���ܶȴ�ļӵ��ܶ�С��Һ���У���ע��ɢ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ


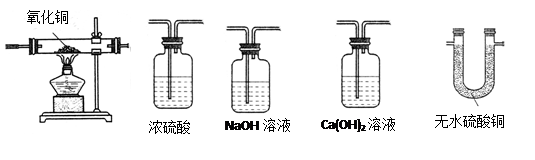 A B C D E
A B C D E װ�õ�����
װ�õ����� ʵ��ǰ ʵ���
ʵ��ǰ ʵ���


