��Ŀ����
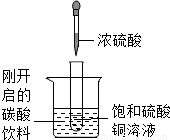
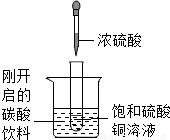
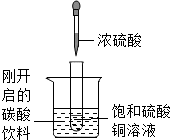 ��ͼ��ʾ�����Թ�С�ķ���ʢ�У�20�棩̼�����ϵ��ձ��У��Թ����ȷ���������������ͭ��Һ�����õιܵμ�5mlŨ�������Թ��У��Իش��������⣺
��ͼ��ʾ�����Թ�С�ķ���ʢ�У�20�棩̼�����ϵ��ձ��У��Թ����ȷ���������������ͭ��Һ�����õιܵμ�5mlŨ�������Թ��У��Իش��������⣺��1��ʵ���пɹ۲쵽��������
��
С�Թ����о�������
С�Թ����о�������
����
�ձ��в�����������
�ձ��в�����������
���ձ������������ԭ��������Ũ������������ͭ��Һ���ų��������ȣ���ʹ�ձ��������¶����ߣ�̼��ֽ����������̼
����Ũ������������ͭ��Һ���ų��������ȣ���ʹ�ձ��������¶����ߣ�̼��ֽ����������̼
����2��ʵ���б��ֳ�Ũ�����
��ˮ��
��ˮ��
�ԣ��ձ�����������Ӧ�Ļ�ѧ����ʽΪH2CO3
H2O+CO2��
| ||
H2CO3
H2O+CO2��
��
| ||
��������1�����ݱ�����Һ�Ͳ�������Һ���ת���ķ������
��2������Ũ���ἰ̼������ʻش�
��2������Ũ���ἰ̼������ʻش�
����⣺��1������Ũ���������ˮ�ԣ�����Ũ�����������ͭ��Һ��ʱ����������Һ�е�ˮ��ʹ��������ͭ��Һ�е�ˮ���٣�������Һ���о��������������ΪŨ��������ˮ��ų��������ȣ�ʹ�ձ���̼�����ϵ��¶����ߣ�̼��ֽ����������̼�����������������ݣ�
��2������ͭ��Һ����������˵����Ũ�������ˮ�ԣ����ݻ�ѧ����ʽ����д���裬����ʽΪ��H2CO3
H2O+CO2����
�ʴ�Ϊ����1����С�Թ����о����������ձ��в����������ݣ�����Ũ������������ͭ��Һ���ų��������ȣ���ʹ�ձ��������¶����ߣ�̼��ֽ����������̼��
��2����ˮ�ԣ�H2CO3
H2O+CO2��
��2������ͭ��Һ����������˵����Ũ�������ˮ�ԣ����ݻ�ѧ����ʽ����д���裬����ʽΪ��H2CO3
| ||
�ʴ�Ϊ����1����С�Թ����о����������ձ��в����������ݣ�����Ũ������������ͭ��Һ���ų��������ȣ���ʹ�ձ��������¶����ߣ�̼��ֽ����������̼��
��2����ˮ�ԣ�H2CO3
| ||
�������������ñ�����Һ�벻������Һ���ת����������Ũ��������Ժ�̼��IJ��ȶ����ǽ������Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
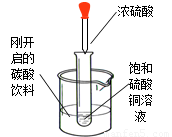
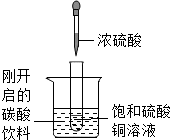 ��ͼ��ʾ�����Թ�С�ķ���ʢ�У�20�棩̼�����ϵ��ձ��У��Թ����ȷ���������������ͭ��Һ�����õιܵμ�5mlŨ�������Թ��У��Իش��������⣺
��ͼ��ʾ�����Թ�С�ķ���ʢ�У�20�棩̼�����ϵ��ձ��У��Թ����ȷ���������������ͭ��Һ�����õιܵμ�5mlŨ�������Թ��У��Իش��������⣺