��Ŀ����
ʵ������ȡ��������װ������ͼ��ʾ��
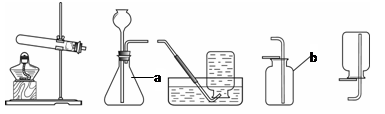
A B C D E
��ش��������⣺
��1��װ���бꡰa����b�����������Ʒֱ���_____ �� ��
��2������װ��A��C������ȡ���������ռ���������Ӧ�� ���� ��
��3����װ��B�� ���ӿ�����ȡ������̼�����װ��B�����Եķ�����____ .
��4��ʵ���ҿ�������ˮ�����ƺͼ�ʯ�����ֹ�̬ҩƷ������ĥ���Ȼ�Ϻ�װ�뷴Ӧװ���У����Ȳ����������塣��ʵ���ѡ�õ����巢��װ��Ϊ ������ĸ��ţ���������
��5����25gʯ��ʯ��Ʒ����������ϡ�����У�ʯ��ʯ�е�̼���������ǡ����ȫ��Ӧ�����ʲ���Ӧ��Ҳ���ܽ⣩���������������Ϊ8.8g����ʯ��ʯ��̼��Ƶ���������Ϊ���٣�

A B C D E
��ش��������⣺
��1��װ���бꡰa����b�����������Ʒֱ���_____ �� ��
��2������װ��A��C������ȡ���������ռ���������Ӧ�� ���� ��
��3����װ��B�� ���ӿ�����ȡ������̼�����װ��B�����Եķ�����____ .
��4��ʵ���ҿ�������ˮ�����ƺͼ�ʯ�����ֹ�̬ҩƷ������ĥ���Ȼ�Ϻ�װ�뷴Ӧװ���У����Ȳ����������塣��ʵ���ѡ�õ����巢��װ��Ϊ ������ĸ��ţ���������
��5����25gʯ��ʯ��Ʒ����������ϡ�����У�ʯ��ʯ�е�̼���������ǡ����ȫ��Ӧ�����ʲ���Ӧ��Ҳ���ܽ⣩���������������Ϊ8.8g����ʯ��ʯ��̼��Ƶ���������Ϊ���٣�
(1) ��ƿ ����ƿ ��2�֣�
(2) �ѵ����ܴ�ˮ�����ó� Ϩ��ƾ��ƣ�2�֣�
(3) D��1�֣� �õ��ɼм�ס�ܣ���©����ע��ˮ��������©�����γ�һ���ȶ���ˮ����˵��װ�ò�©����2�֣�
(4) A ��1�֣� ��Ӧ���ǹ��壬��Ӧ����ȣ�2�֣�
(5) ��6�֣�
�⣺��μӷ�Ӧ̼��Ƶ�����Ϊx
CaCO3��2HCl��CaCl2��H2O��CO2��
100 44
x 8.8 g
100 :44=x:8.8 g
x= 20g
ʯ��ʯ��̼��Ƶ���������Ϊ��
20g/25g��100��=80��
��ʯ��ʯ��̼��Ƶ���������Ϊ80��
(2) �ѵ����ܴ�ˮ�����ó� Ϩ��ƾ��ƣ�2�֣�
(3) D��1�֣� �õ��ɼм�ס�ܣ���©����ע��ˮ��������©�����γ�һ���ȶ���ˮ����˵��װ�ò�©����2�֣�
(4) A ��1�֣� ��Ӧ���ǹ��壬��Ӧ����ȣ�2�֣�
(5) ��6�֣�
�⣺��μӷ�Ӧ̼��Ƶ�����Ϊx
CaCO3��2HCl��CaCl2��H2O��CO2��
100 44
x 8.8 g
100 :44=x:8.8 g
x= 20g
ʯ��ʯ��̼��Ƶ���������Ϊ��
20g/25g��100��=80��
��ʯ��ʯ��̼��Ƶ���������Ϊ80��
�������ع�ʵ������ȡ�����Ͷ�����̼��ԭ����װ�á�ʵ�鲽���ע������Ը�����н���Լ����ø��ݻ�ѧ����ʽ���м���IJ�����㣮
��𣺽⣺��1��a��b�dz��л�ѧ����IJ���������
��2��A�Ǽ����͵�װ�ã���ȡ����ʱΪ�˷�ֹˮ����ը���Թܣ�ʵ����������Ƴ�������Ϩ��ƾ��ƣ�
��3��������̼�ܶȴ��ڿ����������ſ�������ѡ��Dװ�ã����װ��B�����ԣ�����������裺�õ��ɼм�ס�ܣ���©����ע��ˮ��������©�����γ�һ���ȶ���ˮ����˵��װ�ò�©��
��4����Ӧ���ǡ���ˮ�����ƺͼ�ʯ�����ֹ�̬ҩƷ���������ǡ����ȡ����������ѧ���ݿ�֪Aװ�ÿ�����ȡ���飻
��5�����ݻ�ѧ����ʽ���㲽�裬һһ���н�ɣ�
����ȷ�𰸣�
��1����ƿ������ƿ
��2���ѵ����ܴ�ˮ�����ó� Ϩ��ƾ���
��3��D �õ��ɼм�ס�ܣ���©����ע��ˮ��������©�����γ�һ���ȶ���ˮ����˵��װ�ò�©��
��4��A ��Ӧ���ǹ��壬��Ӧ�����
��5���⣺��μӷ�Ӧ̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 8.8 g
100��44=x��8.8 g
x=20g
ʯ��ʯ��̼��Ƶ���������Ϊ��
20g/25g��100%=80%
��ʯ��ʯ��̼��Ƶ���������Ϊ80%
��𣺽⣺��1��a��b�dz��л�ѧ����IJ���������
��2��A�Ǽ����͵�װ�ã���ȡ����ʱΪ�˷�ֹˮ����ը���Թܣ�ʵ����������Ƴ�������Ϩ��ƾ��ƣ�
��3��������̼�ܶȴ��ڿ����������ſ�������ѡ��Dװ�ã����װ��B�����ԣ�����������裺�õ��ɼм�ס�ܣ���©����ע��ˮ��������©�����γ�һ���ȶ���ˮ����˵��װ�ò�©��
��4����Ӧ���ǡ���ˮ�����ƺͼ�ʯ�����ֹ�̬ҩƷ���������ǡ����ȡ����������ѧ���ݿ�֪Aװ�ÿ�����ȡ���飻
��5�����ݻ�ѧ����ʽ���㲽�裬һһ���н�ɣ�
����ȷ�𰸣�
��1����ƿ������ƿ
��2���ѵ����ܴ�ˮ�����ó� Ϩ��ƾ���
��3��D �õ��ɼм�ס�ܣ���©����ע��ˮ��������©�����γ�һ���ȶ���ˮ����˵��װ�ò�©��
��4��A ��Ӧ���ǹ��壬��Ӧ�����
��5���⣺��μӷ�Ӧ̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 8.8 g
100��44=x��8.8 g
x=20g
ʯ��ʯ��̼��Ƶ���������Ϊ��
20g/25g��100%=80%
��ʯ��ʯ��̼��Ƶ���������Ϊ80%

��ϰ��ϵ�д�
�����Ŀ




