��Ŀ����
| pH | Ca2+��Mg2+���� | ϸ������ |
| 6.5��8.5 | ��2.709��1021��?L-1? | ��100��?mL-1? |

��1��ij����ˮ����ȡԭˮ�к��϶��CaCl2��MgCl2��Ca��HCO3��2 �ȣ����������ʯ��ʱһ���������з��������ɻ�ѧ��Ӧ����Mg��HCO3��2+Ca��OH��2=MgCO3��+CaCO3��+2H2O��д��������ѧ��Ӧ�Ļ�ѧ����ʽ��
��2����������ԭˮ���������е������ǣ�
��3��С��ͬѧ�ι۸�����ˮ������ؼ�һƿԭˮ��һƿ����ˮ����æ���������ϱ�ǩ����С�����ü��г������ʺܿ콫��ƿˮ�����������ѡ�õ�������
��2����������ԭˮ���������е�����������ˮ����������ʹ֮������ͨ��CO2��Ŀ���dz�ȥ������Ca��OH��2�͵���pH��
��3������ԭˮ��Ӳ�ȴ���н��
��2����������ԭˮ���������е�����������ˮ����������ʹ֮������ͨ��CO2��Ŀ���dz�ȥ������Ca��OH��2�͵���pH��
��3��ԭˮ��Ӳ�ȴ��������ü��г������ʷ���ˮ����
�ʴ�Ϊ����1��CaO+H2O=Ca��OH��2��
��2������ˮ����������ʹ֮��������ȥ������Ca��OH��2������pH��
��3������ˮ��

ԭˮ��ȡ����Ȼˮ�����ˮˮ�壬������������������������ˮ��ȣ�������ˮˮԴ��ˮ��ͨ��ԭˮ���н϶�Ŀ����Ը�þ��������ҹ�����ˮ�������涨��������±���Ҫ��
| pH | Ca2+��Mg2+���� | ϸ������ |
| 6.5��8.5 | ��2.709��1021��?L-1? | ��100��?mL-1? |
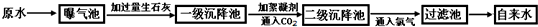
��1��ij����ˮ����ȡԭˮ�к��϶��CaCl2��MgCl2��Ca��HCO3��2 �ȣ����������ʯ��ʱһ���������з��������ɻ�ѧ��Ӧ����Mg��HCO3��2+Ca��OH��2=MgCO3��+CaCO3��+2H2O��д��������ѧ��Ӧ�Ļ�ѧ����ʽ��________������д1����
��2����������ԭˮ���������е������ǣ�________��ͨ��CO2��Ŀ����________��________��
��3��С��ͬѧ�ι۸�����ˮ������ؼ�һƿԭˮ��һƿ����ˮ����æ���������ϱ�ǩ����С�����ü��г������ʺܿ콫��ƿˮ�����������ѡ�õ�������________��
ԭˮ��ȡ����Ȼˮ�����ˮˮ�壬������������������������ˮ��ȣ�������ˮˮԴ��ˮ��ͨ��ԭˮ���н϶�Ŀ����Ը�þ��������ҹ�����ˮ�������涨��������±���Ҫ��
| pH | Ca2+ ��Mg2+���� | ϸ������ |
| 6.5��8.5 | �� 2.709��1021��·L-1 | ��100��·mL-1 |
������ԭˮ����������ˮ�Ĺ�������ʾ��ͼ��
![]()
��1��ij����ˮ����ȡԭˮ�к��϶��CaCl2 ��MgCl2 ��Ca��HCO3��2 �ȣ����������ʯ��ʱһ���������з��������ɻ�ѧ��Ӧ����Mg��HCO3��2+Ca(OH)2=MgCO3��+CaCO3��+2H2O��д��������ѧ��Ӧ�Ļ�ѧ����ʽ�� ������д1����
��2����������ԭˮ���������е������ǣ� ��ͨ��CO2��Ŀ���� �� ��
��3��С![]() ��ͬѧ�ι۸�����ˮ������ؼ�һƿԭˮ��һƿ����ˮ����æ���������ϱ�ǩ����С�����ü��г������ʺܿ콫��ƿˮ�����������ѡ�õ������� ��
��ͬѧ�ι۸�����ˮ������ؼ�һƿԭˮ��һƿ����ˮ����æ���������ϱ�ǩ����С�����ü��г������ʺܿ콫��ƿˮ�����������ѡ�õ������� ��
ԭˮ��ȡ����Ȼˮ�����ˮˮ�壬������������������������ˮ��ȣ�������ˮˮԴ��ˮ��ͨ��ԭˮ���н϶�Ŀ����Ը�þ��������ҹ�����ˮ�������涨��������±���Ҫ��
| pH | Ca2+ ��Mg2+���� | ϸ������ |
| 6.5��8.5 | �� 2.709��1021��·L-1 | ��100��·mL-1 |
������ԭˮ����������ˮ�Ĺ�������ʾ��ͼ��
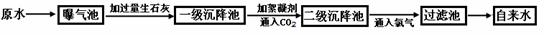
��1��ij����ˮ����ȡԭˮ�к��϶��CaCl2 ��MgCl2 ��Ca��HCO3��2 �ȣ����������ʯ��ʱһ���������з��������ɻ�ѧ��Ӧ����Mg��HCO3��2+Ca(OH)2=MgCO3��+CaCO3��+2H2O��д��������ѧ��Ӧ�Ļ�ѧ����ʽ�� ������д1����
��2����������ԭˮ���������е������ǣ� ��ͨ��CO2��Ŀ���� �� ��
��3��С��ͬѧ�ι۸�����ˮ������ؼ�һƿԭˮ��һƿ����ˮ����æ���������ϱ�ǩ����С�����ü��г������ʺܿ콫��ƿˮ�����������ѡ�õ������� ��
| pH | Ca2+��Mg2+���� | ϸ������ |
| 6.5��8.5 | ��2.709×1021��?L-1? | ��100��?mL-1? |

��1��ij����ˮ����ȡԭˮ�к��϶��CaCl2��MgCl2��Ca��HCO3��2 �ȣ����������ʯ��ʱһ���������з��������ɻ�ѧ��Ӧ����Mg��HCO3��2+Ca��OH��2=MgCO3��+CaCO3��+2H2O��д��������ѧ��Ӧ�Ļ�ѧ����ʽ��______������д1����
��2����������ԭˮ���������е������ǣ�______��ͨ��CO2��Ŀ����______��______��
��3��С��ͬѧ�ι۸�����ˮ������ؼ�һƿԭˮ��һƿ����ˮ����æ���������ϱ�ǩ����С�����ü��г������ʺܿ콫��ƿˮ�����������ѡ�õ�������______��