��Ŀ����
ij��ѧ��ȤС��Ϊ�ⶨ����ʯ��ʯ�Ĵ��ȣ�����������ʵ�飺ȡ12.5gʯ��ʯ��Ʒ�����ձ��У��Ƶ��ձ�����ʢʯ��ʯ��Ʒ��������Ϊ158.0g���ٰ�100gϡ����ƽ���ֳ���ݣ����μ�����Ʒ�У�ÿ�ξ�����ַ�Ӧ������ձ�����ʢ������������ʯ��ʯ�е����ʲ�����ˮ��Ҳ����ˮ�����ᷢ����ѧ��Ӧ����ʵ�����ݼ�¼���£�
����ݴ˷������㣺
��1����һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������______g��
��2��ʵ���в���������̼����������______g��
��3��ͨ�����������ʯ��ʯ��Ʒ��̼��Ƶ����������������ȷ��0.1%����
| ��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� | ����� |
| �ձ�����ʢ����������/g | 176.2 | 194.4 | 213.6 | 233.6 | 253.6 |
��1����һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������______g��
��2��ʵ���в���������̼����������______g��
��3��ͨ�����������ʯ��ʯ��Ʒ��̼��Ƶ����������������ȷ��0.1%����
��1����һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼�������ǣ�158g+20g-176.2g=1.8g��
��2��ʵ���в���������̼���������ǣ�158g+100g-253.6g=4.4g��
��3�����ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx��
CaCO3 +2HCl=CaCl2 +H2O+CO2��
10044
x4.4g
=
x=10g
��100%=80%
�𣺣�1����һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������1.8g��
��2��ʵ���в���������̼����������4.4g��
��3����ʯ��ʯ��Ʒ��̼��Ƶ���������80%��
��2��ʵ���в���������̼���������ǣ�158g+100g-253.6g=4.4g��
��3�����ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx��
CaCO3 +2HCl=CaCl2 +H2O+CO2��
10044
x4.4g
| 100 |
| x |
| 44 |
| 4.4g |
x=10g
| 10g |
| 12.5g |
�𣺣�1����һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������1.8g��
��2��ʵ���в���������̼����������4.4g��
��3����ʯ��ʯ��Ʒ��̼��Ƶ���������80%��

��ϰ��ϵ�д�
�����Ŀ
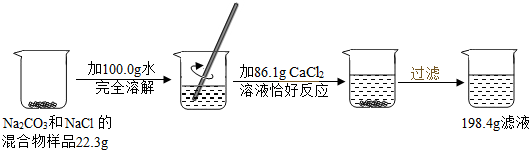
 ��Ӧ������Ҫ����ʯ��ʯ���ٿˣ�
��Ӧ������Ҫ����ʯ��ʯ���ٿˣ�