��Ŀ����
����Ŀ�����Ʋ�ϡ��һ������������Na2SO4��Һ��
��1������50 g��������Ϊ6%��Na2SO4��Һ��
�ټ��㣺��ҪNa2SO4 3.0 g��ˮ47.0 g��
�ڳ�������������ƽ����3.0 g��Na2SO4����ƽ����ֱ�����ƽ�������̷���������ͬ��ֽƬ����________________��Ȼ��________________����������ƽǡ��ƽ�⡣
����ȡ������Ͳ��ȡ47.0 mLˮ��������ͼ�л���47.0 mLˮ��Һ��λ�á�________
![]()
���ܽ⡣
��2��ϡ����Һ���������������ƹ�������Һ��ϡ�����ܶȿɽ��ƿ���1 g/mL��
��ȡ1 mL6%��Na2SO4��Һ��ˮϡ����100 mL���õ���Һa��
������3.0 g Na2SO4��������ҺaŨ����ͬ����Һ���������________mL��
���𰸡� ����������3 g���� ������������ҩƷ ͼ�� 5 000
����������1���ڳ�ȡһ��������ҩƷ���������̷����룬Ȼ��������������ҩƷ��������ƽǡ��ƽ�⡣������Ͳ��ȡҺ�壬����ʱ�������밼Һ�����ʹ�����ˮƽ����2��1 mL6%��Na2SO4��Һ������Ϊ��1 mL��1 g/mL=1g��1mL6%��Na2SO4��Һ�����ʵ�����Ϊ��1g��6%= 0.06 g����Һa�����ʷ���Ϊ��![]() ��100%=0.06%������3.0 g Na2SO4��������ҺaŨ����ͬ����Һ��������x��3.0 g= x��0.06%�����x=5000g���������5000mL��
��100%=0.06%������3.0 g Na2SO4��������ҺaŨ����ͬ����Һ��������x��3.0 g= x��0.06%�����x=5000g���������5000mL��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����Ƹ�����õķ��ͷ۵���Ҫ�ɷ�֮һ��̼�����ơ�ij��ѧ��ȤС���̼�����ƵĻ�ѧ���ʽ���̽����
��̽��ʵ��һ��̼��������Һ������ԣ���pH��ֽ���̼��������Һ��pHԼΪ10.�ɴ˵ó��Ľ�����̼��������Һ��_______��
��̽��ʵ����� ̼�����Ƶ����ȶ��ԣ�ȡһ����̼�����Ʒ���ͭƬ�ϼ��ȣ���ͼ��ʾ��
���������ϡ�̼�����������ֽ⣬����ˮ��������̼��һ�ֳ����Ĺ������ʡ�
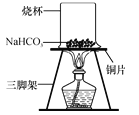
��1������һ��ʱ��۲쵽�ձ������ˮ�顣
��2����ּ��Ⱥ��ձ�Ѹ�ٵ�ת���������������ij���ʯ��ˮ�����۲쵽_____��
��3������ȤС���ͬѧ��Ϊ��ּ��Ⱥ�Ĺ�����������NaOH��Na2CO3��
�����Dz����������_______��
�������ʵ�飬���鷴Ӧ��Ĺ��������NaOH��Na2CO3��������±�����ѡ�Լ���������ϡ���ᡢ�Ȼ����ܹ�������ʯ��ˮ����̪��Һ������ˮ���Թܡ���ͷ�ιܡ�
��� | ʵ����� | Ԥ������ | ���� |
I | ȡ�������Ⱥ�Ĺ����������Թ�A�У�������������ˮ��������ܽ��������______������ֹ�� | ������ɫ���� | ���ﺬNa2CO3 |
II | ȡI��������ϲ���Һ���Թ�B�У��μӷ�̪��Һ | ______ | ���ﲻ��NaOH |