��Ŀ����
 2008��9��27�գ�̫�յ�һ�������ˡ��й��˵Ľ�ӡ�����ҹ����Ƶĺ���Ա�����Ϊ����Ա�ɹ�����̫�������ṩ�˿ɿ��ı�֤��
2008��9��27�գ�̫�յ�һ�������ˡ��й��˵Ľ�ӡ�����ҹ����Ƶĺ���Ա�����Ϊ����Ա�ɹ�����̫�������ṩ�˿ɿ��ı�֤����1�������ߡ����������족�������������ɣ����ڵ�������Ϊ������������紦����������ɵ����ʲ㡢�ϳ����ʵصı������ܲ㡢���Ϲؽڽṹ��ɵ������ܲ㡢�������ϵ����Ʋ㡢���Ȳ����������㣮��������Ȼ�л��߷��Ӳ�����ɵIJ���
A�����ʲ㣻B���������ܲ㣻C�������ܲ㣻D�����Ʋ�
��2������Ա������з�������ϵͳ���������������������裺
��һ�����÷�������һ��װ��ľ̿�ĺ��ӳ�ȥ��������һ����������ľ̿��
�ʶ���������������ﮣ�LiOH�������ռ���ȥ������̼��������﮺��������ƶ��Ǽ�������ƵĻ�ѧ���ʣ���д������������ն�����̼�Ļ�ѧ����ʽ
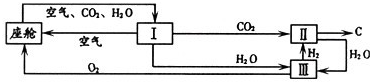
��3����������������ڿ������¹�������ͼ��ʾ��
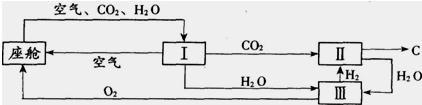
����װ��I�������Ƿ��������ˮ�Ͷ�����̼
��װ�â���CO2��H2�ķ�Ӧװ�ã��÷�Ӧ�Ļ�ѧ����ʽΪ
���ɲ�д����Ӧ��������װ�â�����Ӧ�Ļ�ѧ����ʽΪ
�ڴ�װ��I����ɿ�����O2����Դ��CO2��H2O��������448 g O2������506g CO2����ͬʱ����H2O
��������1�����ݲ��ϵķ�����ֲ��ϵIJ�ͬ�ص������������ȷ���
��2����ľ̿�д��������ɿף�ʹ�������нϸߵıȱ���������ҿ��ڽ������ʱ��ų����кܺõ��������ܣ�
�ڸ������������������̼��Ӧ��������Ϳɵó�����������ն�����̼�Ļ�ѧ����ʽ��
��3���ٸ���ͼʾ��֪��CO2��H2�ķ�Ӧ��������C��H2O��װ�â���ֻ��ˮ���ݴ�д����ѧ����ʽ���ɣ�
�ڸ��ݶ�����̼����Ԫ�ص����������Ͷ�����̼���������Ϳɼ��������506g CO2����������������Ȼ������ʣ�������������H2O����Ԫ�ص������������Ϳɼ����ͬʱ����H2O��������
��2����ľ̿�д��������ɿף�ʹ�������нϸߵıȱ���������ҿ��ڽ������ʱ��ų����кܺõ��������ܣ�
�ڸ������������������̼��Ӧ��������Ϳɵó�����������ն�����̼�Ļ�ѧ����ʽ��
��3���ٸ���ͼʾ��֪��CO2��H2�ķ�Ӧ��������C��H2O��װ�â���ֻ��ˮ���ݴ�д����ѧ����ʽ���ɣ�
�ڸ��ݶ�����̼����Ԫ�ص����������Ͷ�����̼���������Ϳɼ��������506g CO2����������������Ȼ������ʣ�������������H2O����Ԫ�ص������������Ϳɼ����ͬʱ����H2O��������
����⣺��1��û�����˹��ϳɼӹ���ֱ�Ӵ���Ȼ���ȡ�ĸ߷��Ӳ��Ͻ���Ȼ�߷��Ӳ��ϣ�
A�����ʲ���������ɵģ�������Ȼ�߷��Ӳ��ϣ���A�ʺϣ�
B���ϳ������ںϳ��л��߷��Ӳ��ϣ���B���ʺϣ�
C�����Ϲؽڽṹ��ɵ������ܲ����ڷ��ϲ��ϣ���C���ʺϣ�
D��������һ�ֺϳ���ά�����ںϳɲ��ϣ���D���ʺϣ�
��ѡA��
��2����ľ̿��ľ�Ļ�ľ��ԭ�Ͼ�������ȫȼ�գ������ڸ����������������Ƚ⣬�����������ɫ���ɫ�����ȼ�ϣ�ľ̿�д��������ɿף�ʹ�������нϸߵıȱ���������ҿ��ڽ������ʱ��ų����кܺõ��������ܣ����÷�������һ��װ��ľ̿�ĺ��ӳ�ȥ��������һ����������ľ̿�������ԣ�
������������ն�����̼�Ļ�ѧ����ʽΪ��CO2+2LiOH=Li2CO3+H2O��
��3����CO2��H2��Ӧ�Ļ�ѧ����ʽΪ��CO2+2H2=C+2H2O��
װ�â���ֻ��ˮ��ˮ���Ļ�ѧ����ʽΪ��2H2O
2H2��+O2����
������506g CO2����������������506g��
=368g��
��ͬʱ����H2O������Ϊ����448g-368g����
=90g��
�ʴ�Ϊ����1��A��
��2��������CO2+2LiOH=Li2CO3+H2O��
��3����CO2+2H2=C+2H2O��2H2O
2H2��+O2������90��
A�����ʲ���������ɵģ�������Ȼ�߷��Ӳ��ϣ���A�ʺϣ�
B���ϳ������ںϳ��л��߷��Ӳ��ϣ���B���ʺϣ�
C�����Ϲؽڽṹ��ɵ������ܲ����ڷ��ϲ��ϣ���C���ʺϣ�
D��������һ�ֺϳ���ά�����ںϳɲ��ϣ���D���ʺϣ�
��ѡA��
��2����ľ̿��ľ�Ļ�ľ��ԭ�Ͼ�������ȫȼ�գ������ڸ����������������Ƚ⣬�����������ɫ���ɫ�����ȼ�ϣ�ľ̿�д��������ɿף�ʹ�������нϸߵıȱ���������ҿ��ڽ������ʱ��ų����кܺõ��������ܣ����÷�������һ��װ��ľ̿�ĺ��ӳ�ȥ��������һ����������ľ̿�������ԣ�
������������ն�����̼�Ļ�ѧ����ʽΪ��CO2+2LiOH=Li2CO3+H2O��
��3����CO2��H2��Ӧ�Ļ�ѧ����ʽΪ��CO2+2H2=C+2H2O��
װ�â���ֻ��ˮ��ˮ���Ļ�ѧ����ʽΪ��2H2O
| ||
������506g CO2����������������506g��
| 32 |
| 44 |
��ͬʱ����H2O������Ϊ����448g-368g����
| 16 |
| 18 |
�ʴ�Ϊ����1��A��
��2��������CO2+2LiOH=Li2CO3+H2O��
��3����CO2+2H2=C+2H2O��2H2O
| ||
������������Ҫ����ѧ��������ѧ��ѧ֪ʶ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ