��Ŀ����
����Ŀ����������������������ɻ�C919һ�ɳ��죬�к���ҵ������Ϊ�ҹ����������ƵĴ��Ϳͻ�C919����ṹ����������Ҫ�е���4���������������죮�����й������������( )

A. ���������ɻ����죬��Ч�������������ղ�ҵ��������
B. ����ͻ�ʹ�õĸ��ϲ�����Ҫ�л�������̼��ά���ϲ��ϵ�
C. ����ͻ�ʹ�õ�Ӳ������Ҫ�ɷ�������ͭ��þ����
D. �ͻ�ʹ�õ�ȼ�Ϻ���ú����ʯ�����ƵIJ�Ʒ
���𰸡�B
��������
A�������ɻ���Ҫ�IJ����dz����Ӻͷ��࣬���Բ��������ɻ����죬��Ч�������������ղ�ҵ��������������ȷ��
B���л�������һ��������Ʒ�������ڸ��ϲ��ϣ��ʴ���
C��Ӳ������Ҫ�ɷ�������ͭ��þ���裬����ȷ��
D������ú����ʯ�ͷ���IJ�Ʒ֮һ������ȷ��
��ѡ��B

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�������DZ����Ȼ��Դ��
�±��ǿ�������ͼ���Կ�����ú��Ϊԭ�Ϻϳ����أ���ѧʽ��CO(NH2)2��������(���ֲ�������ȥ)���밴Ҫ��ش�������⣺
������� | N | R | Q | CO | ���� |
������� | 78% | 21% | 0.93% | 0.034% | 0.02% |
(1)�ϱ���R��������_____��
(2)����X��H2�ϳɰ����Ļ�ѧ����ʽ��_____���÷�Ӧ����_____��Ӧ(�������Ӧ����)��
(3)��������ij��÷������������֡�
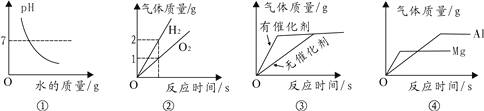
������Һ������������Һ̬��������ʱ���ȷ������������Ƚ϶��ߵķе㣺N2_____O2(ѡ����������������������������)��
���÷���ɸ���롣����ɸ��һ���ڲ��ֲ��о���С���Ĺ��壬ͨ�����Ƶķ���ɸ�ѿ����е������������Ѩ�����������ӷ��룬����Ӵ�С��N2_____O2(ѡ����������������������С����)��
����Ŀ��ͬѧ�Ƕ�һ������������һ��ʱ�����504˫����������ܺ��棬���ʵ�����̽����

��������⣩����ijɷ���ʲô��
���������ϣ�(1)���������������ᷴӦ����Ӧ����Һ��ɫ�ʻ�ɫ������ʽΪFe2O3 +6HCl �T2FeCl3 + 3H2O
(2)�������Ȼ�����Һ�ڳ����·�����Ӧ�����Ȼ���������Ӧ�ķ���ʽΪ��Fe+2FeCl3�T3FeCl2
(3)���������׳���ʯ�ң������ƻ���ˮ��Ӧ���ų��������ȣ�����ʽΪCaO+ H2O�TCa��OH��2
���������룩�����Ϊ���ù����п��ܺ���Fe��CaO��Ca��OH��2��CaCO3
����Ϊ�����ܺ��е�������_____��д��ѧʽ��
��ʵ��̽������ͬѧ�ķ�����
ʵ����� | ʵ������ | ʵ����� |
��1��ȡ������������Թ��У�����������ˮ�ܽ⡣ | �����ܽ�ʱ�Թ���ڷ��̣��Թܵײ��в���� | ������һ������_____�� |
��2����ȡ������������Թ��У��μ�������_____�� | ��������ʧ���д�����ɫ���������������ͨ��ʯ��ˮ��ʯ��ˮ����ǣ����õ�dz��ɫ��Һ�� | ������һ����_____��_____ ʯ��ˮ����ǵķ���ʽ_____ |
��ʵ�����ɣ�ͨ����ͬѧ��ʵ�飬���������ʲ���ȷ��������_____��_____��