��Ŀ����
ʵ���������Ȼ�þ�������ƵĹ���������Ʒ��С��ͬѧ��ⶨ��Ʒ���Ȼ�þ�������������ȳ�ȡ�û������Ʒ20g����ȫ����ˮ�У�Ȼ��ȡ����һ��������������������������Һ100gƽ�����Ĵμ������У������ʵ���������ݼ��±�����������������йؼ��㣺
 | 1 | 2 | 3 | 4 |
| ��������������Һ������/g | 25 | 25 | 25 | 25 |
| ���ɳ���������/g | 2.9 | X | 8.7 | 8.7 |
��2������ԭ����������Ʒ���Ȼ�þ�����������Ƕ��٣�
��3������ʵ�������õ�������������Һ����С��ͬѧ����ʵ���������е�80g������������Ϊ30%������������Һ�����Ƶģ��Լ����������ٿ�ˮ���������ʵ��������������������������������Һ��
��1��5.8g����2��71.25%����3��70g
���������������1�������������ݣ���һ�μ���25g����������Һ����2.9g�������ܹ�����75g����������Һ����8.7g�������ʵڶ��μ���25g����������ҺҲ����2.9g����������x��ֵΪ5.8g��
��2��[��] ��ԭ����������MgCl2������Ϊx��
MgCl2 + 2NaOH = Mg(OH)2�� + 2NaCl
95 58
X 8.7g
95:58=X��8.7g
x=14.25g
ԭ��Ʒ��MgCl2����������Ϊ��14.25/20g ����100% = 71.25%
��ԭ��Ʒ��MgCl2����������Ϊ71.25%��
��3����25g����������Һ�к��������Ƶ�����Ϊy
MgCl2 + 2NaOH = Mg(OH)2�� + 2NaCl
80 58
Y 2.9g
80:58=Y��2.9g
y=4g
NaOH��������������Ϊ4g/25g ��100% = 16%
����Ҫ��ˮ������ΪZ
80g��30% = ��80g + Z���� 16%
Z=70g
����Ҫ��ˮ70g��
���㣺���ݻ�ѧ����ʽ���㣻��Һ����������������
���������ݻ�ѧ����ʽ���㣬Ҫע�����IJ��裬�衢д���ҡ��С��⡢��
��Һ��������������=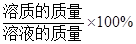 ��
��

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�| �������ʵ����� | 1 | 2 | 3 | 4 |
| ��������������Һ������/g | 25 | 25 | 25 | 25 |
| ���ɳ���������/g | 2.9 | X | 8.7 | 8.7 |
��2������20g����������Ʒ���Ȼ�þ��������

��3����������ʵ�������õ�������������Һ����������������
b�����ձ��м����Ȼ��ƺ�̼�����ƵĹ�������10.0g���ټ���68.9gϡ����ǡ����ȫ��Ӧ��
��Ӧ�������þ�����������ձ���ͬҩƷ����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ���ձ���ͬҩƷ����ʼ������Ϊ165.0g����Ӧ�ķ���ʽΪNaHCO3+HCl=NaCl+H2O+CO2��������
��1����ȫ��Ӧʱ����������̼��������
��2������ԭ��������Ȼ��Ƶ�������
��3������ϡ�������������������
| �������ʵ����� | 1 | 2 | 3 | 4 |
| ��������������Һ������/g | 25 | 25 | 25 | 25 |
| ���ɳ���������/g | 2.9 | X | 8.7 | 8.7 |
��2������ԭ����������Ʒ���Ȼ�þ�����������Ƕ��٣�
��3������ʵ�������õ�������������Һ����С��ͬѧ����ʵ���������е�80g������������Ϊ30%������������Һ�����Ƶģ��Լ����������ٿ�ˮ���������ʵ��������������������������������Һ��
| �� �� ���ʵ����� |
1 | 2 | 3 | 4 |
| ��������������Һ������/g | 25 | 25 | 25 | 25 |
| ���ɳ���������/g | 2.9 | X | 8.7 | 8.7 |
��2������ԭ����������Ʒ���Ȼ�þ�����������Ƕ��٣�
ʵ���������Ȼ�þ�������ƵĹ���������Ʒ��С��ͬѧ��ⶨ��Ʒ���Ȼ�þ�������������ȳ�ȡ�û������Ʒ20g����ȫ����ˮ�У�Ȼ��ȡ����һ��������������������������Һ100gƽ�����Ĵμ������У������ʵ���������ݼ��±�����������������йؼ��㣺
|
| 1 | 2 | 3 | 4 |
| ��������������Һ������/g | 25 | 25 | 25 | 25 |
| ���ɳ���������/g | 2.9 | X | 8.7 | 8.7 |
��1���ϱ���X����ֵΪ_________��
��2������ԭ����������Ʒ���Ȼ�þ�����������Ƕ��٣�����������ػ�ѧ����ʽ���м��㣬д����Ҫ�Ĺ��̣�
��3������ʵ�������õ�������������Һ����С��ͬѧ����ʵ���������е�80g������������Ϊ30%������������Һ�����Ƶģ�ͨ�������֪����� gˮ���������ʵ��������������������������������Һ��
