��Ŀ����
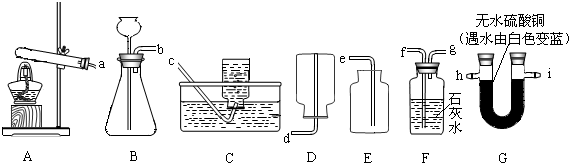
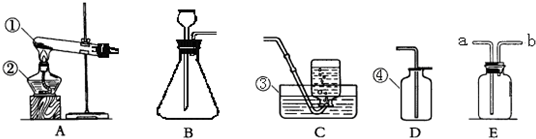
��ͼ��ʾ��ʵ��װ�����dz��ã�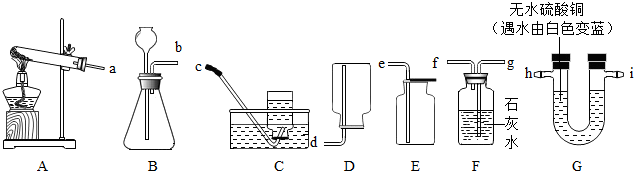
��1��ʵ������ȡ������һ����ѧ����ʽ��
��2�����װ��B�����Եķ�����
��3������Щװ�û����Խ���ʵ��̽�������磺̼����泥�NH4HCO3����һ�ֳ����Ĺ�̬���ʣ�̼����������ֽ�������ֻ��������һ���ǰ�����NH3�����Ҳ�������������������
��������1������ʵ������������ԭ����װ�á��ռ��������н��
��2�����ݼ�������Եķ������н��
��3������̼��������ȷֽ�IJ������̽��
��2�����ݼ�������Եķ������н��
��3������̼��������ȷֽ�IJ������̽��
����⣺��1��ʵ�����ø�������������ķ�Ӧԭ��Ϊ��2KMnO4
K2MnO4+MnO2+O2������װ���ǹ��������A��������������ˮ���ܶȱȿ�����ѡC��E��
��2�����װ��B�����Եķ����ǣ���ֹˮ�мн���Ƥ�ܣ�����©����עˮ���۲�Һ���Ƿ��б仯��
��3��̼��������ȷֽ����ɰ�����ˮ�Ͷ�����̼������ͭ��ˮ�������ʿ�������ͭ����ˮ��������̼��ʹ����ʯ��ˮ����ǣ��ʿ��ó���ʯ��ˮ���������̼��Ҫ�ȼ���ˮ�ټ��������̼�����ȼ��������̼��ͨ������ʯ��ˮʱ�����ˮ������������ԭ�������Ƿ���ˮ��
�ʴ�Ϊ����1��2KMnO4
K2MnO4+MnO2+O2��A��C��E��
��2����ֹˮ�мн���Ƥ�ܣ�����©����עˮ���۲�Һ���Ƿ��б仯��
��3��H2O��CO2��F��G��ahigf��NH4HCO3
NH3��+H2O+CO2��
| ||
��2�����װ��B�����Եķ����ǣ���ֹˮ�мн���Ƥ�ܣ�����©����עˮ���۲�Һ���Ƿ��б仯��
��3��̼��������ȷֽ����ɰ�����ˮ�Ͷ�����̼������ͭ��ˮ�������ʿ�������ͭ����ˮ��������̼��ʹ����ʯ��ˮ����ǣ��ʿ��ó���ʯ��ˮ���������̼��Ҫ�ȼ���ˮ�ټ��������̼�����ȼ��������̼��ͨ������ʯ��ˮʱ�����ˮ������������ԭ�������Ƿ���ˮ��
�ʴ�Ϊ����1��2KMnO4
| ||
��2����ֹˮ�мн���Ƥ�ܣ�����©����עˮ���۲�Һ���Ƿ��б仯��
��3��H2O��CO2��F��G��ahigf��NH4HCO3
| ||
�����������״�����ˮ�Ͷ�����̼�ļ���˳���ȼ���ˮ�ټ��������̼�����dz���Ӧ�ȳ�������̼�ٳ�ˮ��

��ϰ��ϵ�д�
�����Ŀ