��Ŀ����
����Ŀ��������̼�Ĺ����ŷŻ�����ϵ�л������⡣ij��ѧ��ȤС���ͬѧ���������ͼ�ķ���������ת����ҵ�����еĶ�����̼�����ֲ������ԣ���
![]()
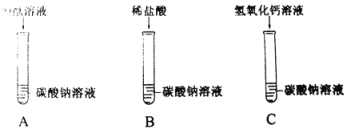
��1�����л���������CO2�йص���_____������ĸ��ţ���
A���� B����ЧӦ C�����ն� D��ɫ��Ⱦ
��2���ڢ��в��ü۸���͵�Ca��OH��2��ֱ������CO2����Ҫԭ����_____��
��3���ڢ�Ӧ�Ļ�ѧ����ʽ��_____��
��4��CaCO3��ҽ���Ͽ�����_____��
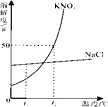
��5����֪����ԭ�ϵļ۸����±���ʾ��
�Լ� | Ca��OH��2 | NaOH |
�۸�Ԫ/Kg�� | 0.36 | 2.90 |
���������У�������ͬ����CO2������ʹ�÷�Ӧ��ȵ��÷�Ӧ������ԭ�ϳɱ����ͣ�ԭ����_____��
���𰸡�B Ca��OH��2����ˮ�������ʵ� Na2CO3+Ca��OH��2��CaCO3��+2NaOH ���Ƽ� ͨ����ӦIIʵ����NaOH��ѭ�����ã�������NaOH�����������Գɱ�����
��������
��1��������̼��������Ի�����ɵ�Ӱ��������ЧӦ�����B��
��2����Ϊ������������ˮ�����յĶ�����̼�����٣��ʵڢ��в��ü۸���͵�Ca��OH��2��ֱ������CO2�����Ca��OH��2����ˮ�������ʵͣ�
��3���ڢ�Ӧ���������ƺ�̼���Ʒ�Ӧ����̼��Ƴ������������ƣ����Na2CO3+Ca��OH��2��CaCO3��+2NaOH��
��4��̼�����ҽ�����������Ƴɲ��Ƽ���������Ƽ���
��5��ͨ����ȡ���̿��Կ�����ͨ����ӦIIʵ����NaOH��ѭ�����ã�������NaOH�����������Գɱ����ͣ����ͨ����ӦIIʵ����NaOH��ѭ�����ã�������NaOH�����������Գɱ����͡�

 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�