��Ŀ����
ij�о�С����������ᾧ����Ʒ�ֽ���ﲢ�ⶨ�������������������ʲ����뷴Ӧ�������ᾧ�壨H2C2O4•2H2O�����������ʼ�����
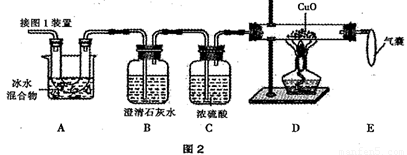
��1�����ȷֽ���ᾧ�������˵�װ���� ����ͼ1��ĸ��ţ���
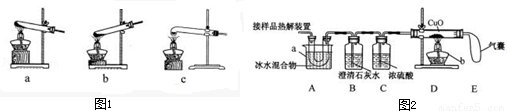
��2��ͼ2����֤�ȷֽ�����к�CO��CO2��װ��
������a��b�����Ʒֱ��� �� ��
��֤������CO2�������� ��֤������CO�������� ��D�з�Ӧ�Ļ�ѧ����ʽ�� ��
��װ��A�������� �����ҵ������� ��
��3��Ϊ�ⶨ��Ʒ�в��ᾧ�����������������������·�����
|
�۵� |
�е� |
���ȶ��� |
��Ӧ |
|
101��C��102��C |
150��C��160��C���� |
100.1��Cʧȥ�ᾧˮ��175��C�ֽ��CO2��CO��H2O |
��Ca��OH��2��Ӧ������ɫ������CaC2O4�� |
�ٳ�һ������Ʒ����ͼװ�ý���ʵ�飬���װ��D��Ӧǰ���������ɴ˼������ʵ������ʵ��ֵƫ�ͣ��ų������Ͳ��������أ���ԭ������У�COδ��ȫ��Ӧ�� ��
�ڳ�ȡ8.75g���ᾧ����Ʒ����50.00g��Һ��ȡ10.00g��Һ��������ϡ���ᣬȻ��μ�25.00g3.16%KMnO4��Һ��ǡ�÷�Ӧ��ȫ��
����֪��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O����KMnO4��Һ�� ɫ��25.00g3.16%KMnO4��Һ��KMnO4������ g���������Ʒ�е�����������[д��������̣�M2��H2C2O4��=90��M2��H2C2O4•2H2O��=126��M2��KMnO4��=158]��
��1��c
��2�����ձ����ƾ���
��B�г����ʯ��ˮ����ǣ�D�к�ɫ�����ɺ�ɫ
CO+CuO Cu+CO2
Cu+CO2
�۳�ȥ������������ֹ�Զ�����̼�ļ���������ţ��ռ�һ����̼����ֹ��Ⱦ����
��3�������ɵ�ͭ�ֱ����� ���Ϻ� 0.79g 90%
��������
�����������1��������۵�ϵͣ����������ۻ�����cװ�ü��Ȳ���ʱ�����������������������Ȳ��ᣮ
���c��
��2��������a��b�����Ʒֱ����ձ����ƾ��ƣ�
����ձ����ƾ��ƣ�
��֤������CO2�������ǣ�B�г����ʯ��ˮ����ǣ�֤������CO�������ǣ�D�к�ɫ�����ɺ�ɫ��
���B�г����ʯ��ˮ����ǣ�D�к�ɫ�����ɺ�ɫ��
D������ͭ��һ����̼�ڼ���ʱ��Ӧ������ͭ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽ�ǣ�CO+CuO Cu+CO2��
Cu+CO2��
���CO+CuO Cu+CO2��
Cu+CO2��
��װ��A�������ǣ���ȥ������������ֹ�Զ�����̼�ļ���������ţ����ҵ������ǣ��ռ�һ����̼����ֹ��Ⱦ������
�����ȥ������������ֹ�Զ�����̼�ļ���������ţ��ռ�һ����̼����ֹ��Ⱦ������
��3����һ����̼���ַ�Ӧ�����ɵ�ͭ���±����������ض��ܹ����¼������ʵ������ʵ��ֵƫ�ͣ�
������ɵ�ͭ�ֱ�������
�ڸ��������Һ����ɫ�Ϻ�ɫ�ģ�
����Ϻ죮
25.00g3.16%KMnO4��Һ��KMnO4������Ϊ��25.00g��3.16%=0.79g��
���0.79��
��10.00g��Һ�к����ᾧ�������ΪX��
��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O��֪��
5H2C2O4•2H2O��5H2C2O4��2KMnO4��
630 316
X 0.79g
 =
=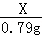
X=1.575g��
50.00g��Һ�к����ᾧ�������Ϊ��1.575g��5=7.875g��
���ᾧ�����������Ϊ��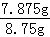 ��100%=90%��
��100%=90%��
����Ʒ�в��ᾧ�����������Ϊ90%��
���㣺ʵ��̽�����ʵ���ɳɷ��Լ���������������ļ�������ӷ�����
�����������漰��ѧ����ʽ����д��ʵ��������жϡ����ݻ�ѧ����ʽ���м���ȷ����֪ʶ���ǵ��͵��ۺ��⣮

 ��У����ϵ�д�
��У����ϵ�д�ij�о�С����������ᾧ����Ʒ�ֽ������ⶨ��Ʒ�в��ᾧ��������������������ʲ����뷴Ӧ������֪��Ũ�������Ϊ����������ᾧ�壨H2C2O4��2H2O �������ʼ��±���
|
�۵� |
�е� |
���ȶ��� |
���� |
|
101�桫102�� |
150�桫160������ |
100.1��ֽ��ˮ��175��ֽ��CO2��CO��H2O |
�� Ca(OH)2��Ӧ������ɫ����(CaC2O4) |
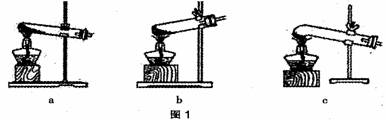
��1��ͼ 1 �Ǽ���װ�á������˵ļ��ȷֽ���ᾧ��װ����C����ѡװ�� a ���ܻ���ɵĺ����_____________________����ѡװ��B���ܻ���ɵĺ����_________________��
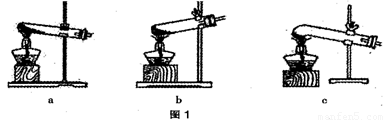
��2��ͼ 2 ����֤�ȷֽ�����к� CO �� CO2��װ�á�
�� װ�� A ��������_____________________�����ҵ�������_____________________��
�� ֤������ CO2��������______________��֤������ CO ��������______________��
��3��Ϊ�ⶨ��Ʒ�в��ᾧ�������������������·�������ȡһ������Ʒ��������װ�ý���ʵ�飬����װ��D��Ӧǰ���������ɴ˼������ʵ������ʵ��ֵƫ�ͣ��ų������Ͳ������أ������ԭ��_________________________________________________��
ij�о�С����������ᾧ����Ʒ�ֽ���ﲢ�ⶨ�������������������ʲ����뷴Ӧ�������ᾧ�壨 H2C2O4•2H2O�����������ʼ�����
��1�����ȷֽ���ᾧ�������˵�װ������ ������ͼ1��ĸ��ţ���

��2��ͼ2����֤�ȷֽ�����к�CO��CO2��װ��
������a��b�����Ʒֱ����� ������ ����
��֤������CO2���������� ����֤������CO���������� ����D�з�Ӧ�Ļ�ѧ����ʽ���� ����
��װ��A���������� �������ҵ��������� ����
��3��Ϊ�ⶨ��Ʒ�в��ᾧ�����������������������·�����
| �۵� | �е� | ���ȶ��� | ��Ӧ |
| 101��C��102��C | 150��C��160��C���� | 100.1��Cʧȥ�ᾧˮ��175��C�ֽ��CO2��CO��H2O | ��Ca��OH��2��Ӧ������ɫ������CaC2O4�� |
�ٳ�һ������Ʒ����ͼװ�ý���ʵ�飬���װ��D��Ӧǰ���������ɴ˼������ʵ������ʵ��ֵƫ�ͣ��ų������Ͳ��������أ���ԭ������У�COδ��ȫ��Ӧ���� ����
�ڳ�ȡ8.75g���ᾧ����Ʒ����50.00g��Һ��ȡ10.00g��Һ��������ϡ���ᣬȻ��μ�25.00g3.16%KMnO4��Һ��ǡ�÷�Ӧ��ȫ��
����֪��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O����KMnO4��Һ
���� ��ɫ��25.00g3.16%KMnO4��Һ��KMnO4�������� ��g���������Ʒ�е�����������[д��������̣�M2��H2C2O4��=90��M2��H2C2O4•2H2O��=126��M2��KMnO4��=158]��
ij�о�С����������ᾧ����Ʒ�ֽ������ⶨ��Ʒ�в��ᾧ��������������������ʲ����뷴Ӧ������֪��Ũ�������Ϊ����������ᾧ�壨H2C2O4��2H2O �������ʼ��±���
| �۵� | �е� | ���ȶ��� | ���� |
| 101�桫102�� | 150�桫160������ | 100.1��ֽ��ˮ��175��ֽ��CO2��CO��H2O | �� Ca(OH)2��Ӧ������ɫ����(CaC2O4) |
��1��ͼ 1 �Ǽ���װ�á������˵ļ��ȷֽ���ᾧ��װ���� c ����ѡװ�� a ���ܻ���ɵĺ����_____________________����ѡװ�� b ���ܻ���ɵĺ����_________________��
��2��ͼ 2 ����֤�ȷֽ�����к� CO �� CO2��װ�á�

�� װ�� A ��������_____________________�����ҵ�������_____________________��
�� ֤������ CO2��������______________��֤������ CO ��������______________��
��3��Ϊ�ⶨ��Ʒ�в��ᾧ�������������������·�������ȡһ������Ʒ��������װ�ý���ʵ�飬����װ�� D ��Ӧǰ���������ɴ˼������ʵ������ʵ��ֵƫ�ͣ��ų������Ͳ������أ������ԭ��_________________________________________________��
