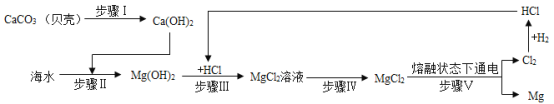
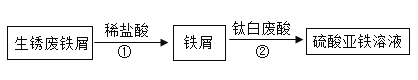
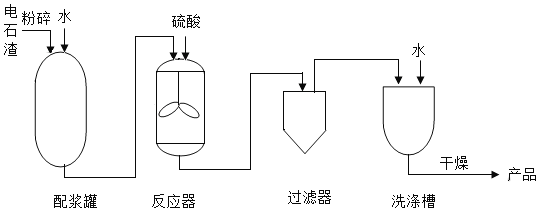
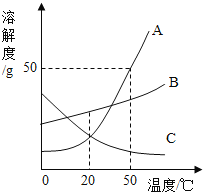
��Ŀ����
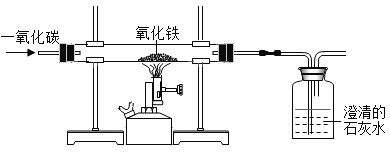
����Ŀ��ijͬѧΪ��֤̿�������в���ȫȼ�յIJ������CO����CO2�� �����ͼ��ʾ��
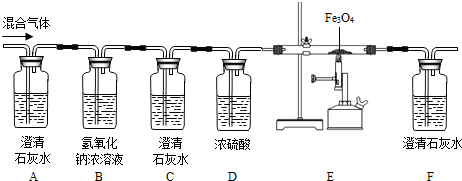
���̽���ʵ�飬������ѧ֪ʶ�ش��������.
��1�����������Ӻ�װ�ã���װ��ҩƷ֮ǰ��������еIJ�����________��
��2��Bװ����NaOHŨ��Һ��������________��
��3��ʵ�鿪ʼʱ��������ͨ��������һ��ʱ���ٵ�ȼ�ƾ���Ƽ��ȣ�Ŀ����________��
��4�����������ͼ���۲쵽________����дʵ������ʱ��֤����������м���CO��CO2��
��5��д��E�еķ�Ӧ��ѧ����ʽ��________��
���𰸡����װ�õ������� ��ȥ��������еĶ�����̼ �ž����ڿ�������ֹ������ը A��F�е�ʯ��ˮ����ǣ�C�е�ʯ��ˮ���������� Fe2O3+3CO![]() 2Fe+3CO2
2Fe+3CO2
��������
��1����װ��װ�ú�װҩƷǰҪ�ȼ��װ�������ԣ�
��2��������̼�����������ܷ�Ӧ����̼���ƺ�ˮ��Bװ��������������Һ�������dz�ȥ��������еĶ�����̼���Է�ֹ����CO����֤��
��3��CO�п�ȼ�ԣ�ʵ�鿪ʼʱ��������ͨ��������һ��ʱ���ٵ�ȼ�ƾ���Ƽ��ȣ����ž����ڿ�������ֹ������ը��
��4����A��F��ʯ��ˮ����ǣ�C��ʯ��ˮ������������ ֤����������м���CO��CO2��
��5��E��Ϊһ����̼���������ķ�Ӧ������ʽΪ Fe2O3+3CO![]() 2Fe+3CO2��
2Fe+3CO2��