��Ŀ����
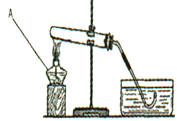
ʵ���Ҳ���������װ����ͼ��ʾ����ش���������
��1������B�����ƣ� ����������;�� ��
��2����������غͶ���������ȡ����������Ϊһ��Ҫ���ӵ������� ��д����������������Ļ�ѧ����ʽ�� ������װ��H�ռ�����������Ӧ��____���a����b�����˵��롣
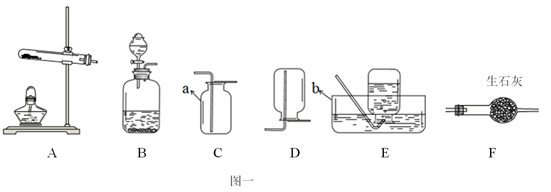
��3����Ҫ��װһ����ȡ������̼�����װ�ã���ѡ���װ�����Ϊ ������ĸ��
��4����ȡ������̼ʱ���������ʯ�е�̼�����73gϡ����ǡ����ȫ��Ӧ�����ʲ����뷴Ӧ��������4��4�˶�����̼�������������ʵ���������Ϊ���٣������ݻ�ѧ����ʽ��ʽ���㣩
��1������©�� ����Һ��ҩƷ
��2���ƾ��� 2KClO3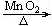 2KCl+3O2�� a
2KCl+3O2�� a
��3��ABCDF ��4��10%
���������������1������������ʶ�ǣ�����©������Ҫ��;������Һ��ҩƷ
��2��ʵ�鷢��װ�õ�ѡ�����ݣ���Ӧ���״̬�ͷ�Ӧ������������غͶ���������ȡ��������Ӧ������Ҫ���ȣ���һ��Ҫ���ӵ������ǣ��ƾ��ƣ���ѧ��Ӧ����ʽΪ��2KClO3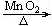 2KCl+3O2��������װ��H�ռ�����������ˮ������������Ӧ��a�ˣ����̹ܣ�����
2KCl+3O2��������װ��H�ռ�����������ˮ������������Ӧ��a�ˣ����̹ܣ�����
��3��ʵ�鷢��װ�õ�ѡ�����ݣ���Ӧ���״̬�ͷ�Ӧ�������ռ�װ�õ�ѡ�������ǣ�����������ܽ��Ժ��ܶȣ�ʵ������ȡ������̼�������ô���ʯ��ϡ�����ڳ����·�Ӧ���Ҷ�����̼���ܶȱȿ�����������ˮ������Ҫ��װһ����ȡ������̼�����װ�ã���ѡ���װ�����ΪABCDF
��4�����ݻ�ѧ����ʽ��CaCO3 +2HCl��CaCl2 +H2O +CO2�� ��HCl�������̼��������ϵ����������������ʵ�������������������������ʵ���������
�⣺�����������ʵ�����Ϊx
CaCO3 +2HCl��CaCl2 +H2O +CO2��
73 44
x 4��4g
73��44=x��4��4g x=7��3g
���������ʵ���������=7��3g/73g��100%=10%
���㣺ʵ��װ�õ�ѡ�����ݣ�ʵ������ȡ������������̼��ԭ�������ݻ�ѧ����ʽ���еļ���

 ��У����ϵ�д�
��У����ϵ�д�