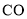��Ŀ����
����ͼ�ļ��ȴ�������Ұ�����ʳ����ȴ��еĹ����ĩ��þ�ۡ����ۺ��Ȼ��ƣ�ʹ��ʱ�����м���ˮ���е�ˮ�����ɲ��������ȡ�ʵ��С����Է��ȹ����еķ�Ӧԭ��չ��̽����
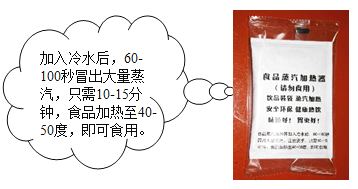
���������ϣ�
������þ������ˮ�����û���Ӧ���ҷ��ȡ�
������ʵ�飩
ͬѧ������ͼ��ʾװ�ý���ģ��ʵ�飺�ֱ�ȡ��ͬ�ɷֵĹ����ĩ������У�ͨ����Һ©�������о�����8 mLˮ����ȡ�������ù�Һ����������¶ȣ�ʵ���¼���±���
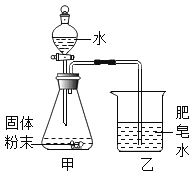
(ʵ��ʱ������Ϊ22.8��)
ʵ����� | A | B | C | D | E | F |
����ɷ� | Mg | Fe | Mg+Fe | Mg+NaCl | Fe+NaCl | Mg+Fe+NaCl |
�������� | ���������ݣ����Ե�ȼ | �� | ���������ݣ����Ե�ȼ | �϶�����ݣ���ȼ�б����� | ������ | ���������ݣ���ȼ�б����� |
������¶� | 23.1�� | 22.8�� | 23.1�� | 24.2�� | 22.8�� | 27.2�� |
����������ۣ�
(1)ʵ��B����������Ϊ_______________��
(2)ʵ��A֤����þ����ˮ�ܷ�Ӧ����ɸ÷�Ӧ�Ļ�ѧ����ʽMg+2H2O=____+H2��
(3)ʹþ����ˮѸ�ٷ�Ӧ�����ȵ���ѷ�������þ���м���_____________��
����˼�Ľ���
(4)ͬѧ�Ƿ���ʵ�����ݷ��֣����ߵ��¶�û�дﵽʳƷ���ȴ���Ч��������ܵ�ԭ����___________��
(5)ͬѧ�Ǹ���ʵ������һ���²⣬ʵ��F�еķ�Ӧ��������ʣ�������������Ȼ�������ۣ����������۴��ڵĵ�ʵ�鷽��Ϊ��ȡ������������___________________��
(6)������ʵ������ó���NaCl����ˮû�������ı仯���Ľ��ۣ����ݵ�����ʵ����(��ʵ�����)______________��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�




 B.
B.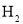 C.
C.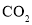 D.
D.