��Ŀ����
����Ŀ����ͼ��ʾijЩ���ʼ�ת����ϵ����������ʾ����֮�����ת����ϵ��������A��B������ͬԪ����ɵ���ɫҺ�壬��A��������ɱ�����ã�C��FΪ���嵥�ʣ�F�ǿ����к�����ߵ����壻D��EΪ���壬D�ʺ���ɫ��E��Ӧ����㷺�Ľ�����G����Է�������Ϊ100�����Ԫ�ص�ԭ�Ӹ�����Ϊ3��2���ش��������⣺
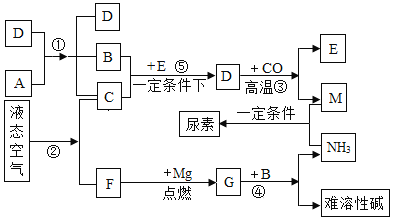
��1������D��������_____________��G�Ļ�ѧʽ��____________��
��2���ڴ�����____________��ͬ������ţ���������������塣
A�е� B�ܶ� C�ܽ���
��3����Ӧ�١��۵Ļ�ѧ����ʽ����___________����___________��
��4��M��NH3����������[CO(NH2)2]����μӷ�Ӧ��M��NH3��������Ϊ_______��
���𰸡����� Mg3N2 A 2H2O2![]() 2 H2O + O2 �� Fe2O3 + 3CO
2 H2O + O2 �� Fe2O3 + 3CO![]() 2Fe + 3CO2 22:17
2Fe + 3CO2 22:17
��������
A��B������ͬԪ����ɵ���ɫҺ�壬��A��������ɱ�����ã�C��FΪ���嵥�ʣ�A�ֽ������B��C������A�ǹ���������Һ��B��ˮ��C��������D�Ƕ������̣�F�ǿ����к�����ߵ����壬����F�ǵ�����E��Ӧ����㷺�Ľ���������E����������������ˮ��һ�����������⣬����D������������������һ����̼�����������Ͷ�����̼������M�Ƕ�����̼��������þ�ڵ�ȼ�����������ɵ���þ������G�ǵ���þ������þ��ˮ��Ӧ����������þ�Ͱ�����������֤���Ƶ���ȷ��
��1�����ж������̵������Ǵ����ã�G�ǵ���þ��
��2���ڴ�����Һ̬�����õ������������������÷е㲻ͬ����ѡ��A��
��3����Ӧ���ǹ��������ڶ������̵Ĵ������·ֽ�����ˮ����������ѧ����ʽΪ��2H2O2![]() 2H2O+O2����
2H2O+O2����
��Ӧ������������һ����̼��Ӧ�������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪFe2O3+3CO![]() 2Fe+3CO2��
2Fe+3CO2��
��4��������̼��NH3����������[CO��NH2��2]����ѧ����ʽΪ��CO2+2NH3 CO��NH2��2+H2O����μӷ�Ӧ�Ķ�����̼��NH3��������Ϊ��44����2��17��=22��17��
CO��NH2��2+H2O����μӷ�Ӧ�Ķ�����̼��NH3��������Ϊ��44����2��17��=22��17��