��Ŀ����
ij�о���ѧϰС��ԡ�SO2�ܷ���H2O��Ӧ�����ᡱ�Ŀ��չ̽�����������Ϻ��֪����SO2��������һ����ɫ���壬������ˮ��
������ʹ��ɫ��ʯ����ֽ��ɺ�ɫ��
��SO2�ж����������������ͼ��ʾװ�ý���ʵ�飮
����������ǵ�̽������ش��й����⣺
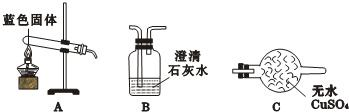
��1��ʵ������У�Aװ������ɫʯ����ֽ����ɫʼ��û�б仯��Aװ�õ������ǣ�______��
��2����ͨ��SO2֮ǰ��Bװ���н�ͷ�ι��ڵ�����ˮ�ε���ɫʯ����ֽ�ϣ�δ����ֽ��ɫ�����仯���ɴ˵ó��Ľ�����______������SO2ͨ��ʱ������ʪ����ɫʯ����ֽ��죬������˵��______��
��3��ʵ�鷽���У���һ�����Ե���©�����������ָ������֮��______��

���𰸡�����������̽���˶�����������ʺͱ仯���ɣ�������Ƶ�ʵ�鲽���Ǣ��ȰѸ���Ķ�������ͨ��������ɫʯ����ֽ�ϣ������Dz���ɫ����Bװ���н�ͷ�ι��ڵ�����ˮ�ε���ɫʯ����ֽ�ϣ�δ����ֽ��ɫ�����仯����ͨ��SO2���壬��ɫʯ����ֽ���ɫ���ɴ˵ó����ۣ�SO2��H2O��Ӧ�����ᣮ��Ҫע��SO2�ж�������Ⱦ������
����⣺��1��Aװ������ɫʯ����ֽ����ɫʼ��û�б仯��˵������Ķ���������ʹ��ʯ����ֽ��ɫ���ʴ�Ϊ��֤��SO2����ʹ��ɫʯ����ֽ���
��2����ͨ��SO2֮ǰ��Bװ���н�ͷ�ι��ڵ�����ˮ�ε���ɫʯ����ֽ�ϣ�δ����ֽ��ɫ�����仯��˵��ˮ����ʹ���ɫ������SO2ͨ��ʱ������ʪ����ɫʯ����ֽ��죬˵������������ˮ��Ӧ�������ᣮ�ʴ�Ϊ��֤��ˮ����ʹ��ɫʯ����ֽ��죨��֤��ˮû�����ԣ���SO2��H2O��Ӧ�����ᣮ
��3���������Ͽ�֪��SO2�ж�������Ⱦ��������˶�β��Ҫ���д������ʴ�Ϊ��β��û�д�������Ӧ��һ��β������װ�ã�
������������̽����SO2�ܷ���H2O��Ӧ�����ᣬ����ʵ��̽���⣬����ʵ����̵�̽�������н��۵�̽��������������⡢�������ϣ�Ȼ�����ʵ�鷽��������ʵ�飬���ó���ȷ�Ľ��ۣ���������ѧ����ʵ�������������ҳ�ʵ��IJ���֮���������ԸĽ�����������Ҫ������ʵ�����У�
����⣺��1��Aװ������ɫʯ����ֽ����ɫʼ��û�б仯��˵������Ķ���������ʹ��ʯ����ֽ��ɫ���ʴ�Ϊ��֤��SO2����ʹ��ɫʯ����ֽ���
��2����ͨ��SO2֮ǰ��Bװ���н�ͷ�ι��ڵ�����ˮ�ε���ɫʯ����ֽ�ϣ�δ����ֽ��ɫ�����仯��˵��ˮ����ʹ���ɫ������SO2ͨ��ʱ������ʪ����ɫʯ����ֽ��죬˵������������ˮ��Ӧ�������ᣮ�ʴ�Ϊ��֤��ˮ����ʹ��ɫʯ����ֽ��죨��֤��ˮû�����ԣ���SO2��H2O��Ӧ�����ᣮ
��3���������Ͽ�֪��SO2�ж�������Ⱦ��������˶�β��Ҫ���д������ʴ�Ϊ��β��û�д�������Ӧ��һ��β������װ�ã�
������������̽����SO2�ܷ���H2O��Ӧ�����ᣬ����ʵ��̽���⣬����ʵ����̵�̽�������н��۵�̽��������������⡢�������ϣ�Ȼ�����ʵ�鷽��������ʵ�飬���ó���ȷ�Ľ��ۣ���������ѧ����ʵ�������������ҳ�ʵ��IJ���֮���������ԸĽ�����������Ҫ������ʵ�����У�

��ϰ��ϵ�д�
 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�
�����Ŀ