��Ŀ����
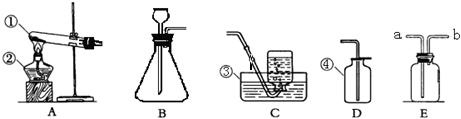
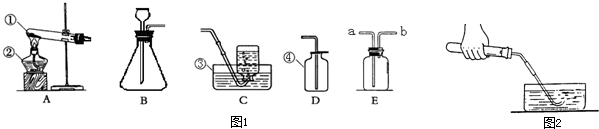
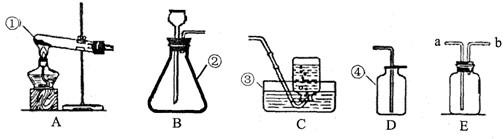
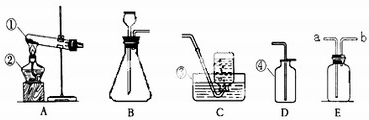
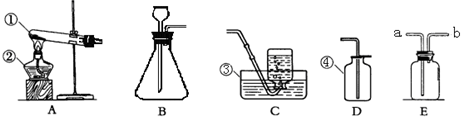
С�ꡢС���ͬѧ������ͼ��ʾ��ʵ��װ����ȡ���ռ�������

��1����д���б�����������ƣ��� ��
�� �� ��4�֣�
��2�����ù���������Һ��ȡ����Ӧѡ�õķ���װ���� (�����)��Ҫ�ռ��ϴ�������
��Ӧѡ�� ����Ӧ�����ֱ���ʽ�� ����Ӧ����
������ ��Ӧ(ѡ����ϡ��ֽ⡱)���������̵������� ��
��3��ʵ�鿪ʼǰ��ͼ��ʾ���װ�������ԣ���� ˵�����������á�

��4��ʵ�����ø��������������װ�����Թܿڷ����ŵ������� ���ø���
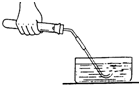
�����ȡ���������ֱ���ʽΪ ��ʵ�����
ʱ�Ƚ������Ƴ�ˮ�棬��Ϩ��ƾ��Ƶ�Ŀ���Ƿ�ֹ________________________����ķ�
����
��5����ѡ��D�ռ�������ԭ���� ��
(20��) (1)�Թܡ��ƾ��ȡ�ˮ�ۡ�����ƿ
(2)B��C��H2O2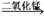 H2O+O2 �ֽⷴӦ��������
H2O+O2 �ֽⷴӦ��������
�ǹܿ������ݲ���
�ȷ�ֹ������ط�ĩ���뵼�ܡ�KMnO4 K2MnO4+MnO2+O2��ˮ��������Թ����ѡ�û��Ԥ�ȵ� �������ܶȱȿ�����
K2MnO4+MnO2+O2��ˮ��������Թ����ѡ�û��Ԥ�ȵ� �������ܶȱȿ�����
����������

 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д�