��Ŀ����
����Ŀ��ʪ��ұͭ���ŷŵķ�ˮ�к������������ͭ������Ⱦ�Ϊ�ⶨ�÷�ˮ�и���Ⱦ��ĺ���������С���ͬѧ����������ʵ�飮ȡ��ˮ500g�������м���������������Ϊ20%������������Һ����ó��������������������������Һ��������ϵ��ͼ�������������ݼ��㣺 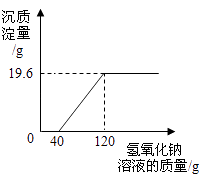
��1��500g��ˮ�м�������������Һ��ַ�Ӧ�õ����������ʵ�����mol��
��2���÷�ˮ������ͭ������������������д��������̣�
��3���÷�ˮ����������ʵ����� ��
���𰸡�
��1��0.2
��2���⣺���ˮ������ͭ�����ʵ���Ϊx�����ĵ��������Ƶ����ʵ���Ϊy��
CuSO4+2NaOH= | Cu��OH��2��+Na2SO4 |
1 | 1 |
x | 0.2mol |
![]() =
= ![]()
x=0.2mol��
�÷�ˮ������ͭ��������������Ϊ ![]() ��100%=6.4%
��100%=6.4%
�𣺸÷�ˮ������ͭ��������������Ϊ6.4%
��3��0.1mol
���������⣺�ٵõ�������ͭ���������ʵ���Ϊ�� ![]() =0.2mol���������ᷴӦ���������Ƶ�����Ϊ40g��20%=8g �����ᷴӦ���������Ƶ����ʵ���Ϊ��
=0.2mol���������ᷴӦ���������Ƶ�����Ϊ40g��20%=8g �����ᷴӦ���������Ƶ����ʵ���Ϊ�� ![]() =0.2mol
=0.2mol
��÷�ˮ����������ʵ�����y
H2SO4+ | 2NaOH=Na2SO4+2H2O |
1 | 2 |
y | 0.2mol |
![]() =
= ![]()
y=0.1mol�𣺢�0.2����6.4%��0.1mol
�����㾫�������ø��ݻ�ѧ��Ӧ����ʽ�ļ������Ŀ�����жϼ��ɵõ��𰸣���Ҫ��֪�����ʼ�������=ϵ������Է�������֮�ȣ�

 ��У����ϵ�д�
��У����ϵ�д�
