��Ŀ����
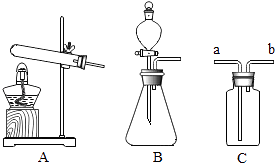
����Ŀ����ѧ�Ƽ�С��ͬѧģ��������������ռ��ʾ��ͼ��ͼ�� 
��ش��������⣮
��д������ʯ��ʯ�Ļ�ѧ����ʽ������2�������� ��
������Ӧ���д���ȫ����Ӧ�����ᾧ�õ��Ĺ����ռ��л��������������ʣ�ԭ���� �� ���������ѡ�õ��Լ��� �� ����ʱ�۲쵽 �� ˵���ᾧ�õ��Ĺ����ռ�ȷʵ�����������ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
����ҺC�ɼ��뷴Ӧ����ѭ�������ã�Ŀ���� ��
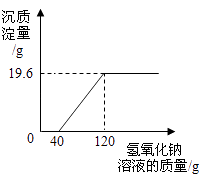
����Ͷ�뷴Ӧ�����еĴ���Ϊnmol����Ϊ��ȷ������ȫ��ת��Ϊ�ռͶ����ʯ�ҵ����ʵ���ȡֵ��Χ�� ��
���𰸡�CaCO3 ![]() CaO+CO2������������ҺB�к��� Ca��OH��2��Na2CO3���а�ɫ�������ɣ�Na2CO3+Ca��OH��2��CaCO3��+2NaOH�����ͳɱ�����ֹ��Ⱦ������CaO�����ʵ�����n mol
CaO+CO2������������ҺB�к��� Ca��OH��2��Na2CO3���а�ɫ�������ɣ�Na2CO3+Ca��OH��2��CaCO3��+2NaOH�����ͳɱ�����ֹ��Ⱦ������CaO�����ʵ�����n mol
���������⣺��̼����ڸ��µ����������������ƺͶ�����̼����ѧ����ʽΪ��CaCO3 ![]() CaO+CO2������������ʹ��Һ�еľ������������Բ���2���������������ڷ�Ӧ���д���ȫ����Ӧ�����ᾧ�õ��Ĺ����ռ��к��з�Ӧʣ����������ƣ��������ƺ�̼���ƻ�����̼��Ƴ������������ƣ����Լ��������ѡ�õ��Լ���Na2CO3 �� ����ʱ�۲쵽�а�ɫ�������ɣ�˵���ᾧ�õ��Ĺ����ռ�ȷʵ�����������ʣ��÷�Ӧ�Ļ�ѧ����ʽΪNa2CO3+Ca��OH��2��CaCO3��+2NaOH���۾�ϴ�ӹ��˺����õ���Һ��һ���Ậ���������ƺ��������ƣ�ֱ���ŷŻᵼ���˷Ѻ���Ⱦ������ѭ�������ã�����Ч�Ľ��������ɱ��ͷ�ֹ������Ⱦ����Ϊ��ʹ̼������ȫ��Ӧ����������Ӧ���ǹ����ģ�1mol�������ƻ�����1mol���������ƣ�����Ͷ����ʯ�ҵ����ʵ���ȡֵ��Χ��CaO�����ʵ�����n mol�� ���Դ��ǣ���CaCO3
CaO+CO2������������ʹ��Һ�еľ������������Բ���2���������������ڷ�Ӧ���д���ȫ����Ӧ�����ᾧ�õ��Ĺ����ռ��к��з�Ӧʣ����������ƣ��������ƺ�̼���ƻ�����̼��Ƴ������������ƣ����Լ��������ѡ�õ��Լ���Na2CO3 �� ����ʱ�۲쵽�а�ɫ�������ɣ�˵���ᾧ�õ��Ĺ����ռ�ȷʵ�����������ʣ��÷�Ӧ�Ļ�ѧ����ʽΪNa2CO3+Ca��OH��2��CaCO3��+2NaOH���۾�ϴ�ӹ��˺����õ���Һ��һ���Ậ���������ƺ��������ƣ�ֱ���ŷŻᵼ���˷Ѻ���Ⱦ������ѭ�������ã�����Ч�Ľ��������ɱ��ͷ�ֹ������Ⱦ����Ϊ��ʹ̼������ȫ��Ӧ����������Ӧ���ǹ����ģ�1mol�������ƻ�����1mol���������ƣ�����Ͷ����ʯ�ҵ����ʵ���ȡֵ��Χ��CaO�����ʵ�����n mol�� ���Դ��ǣ���CaCO3 ![]() CaO+CO2��������������ҺB�к��� Ca��OH��2 �� Na2CO3 �� �а�ɫ�������ɣ�Na2CO3+Ca��OH��2��CaCO3��+2NaOH���۽��ͳɱ�����ֹ��Ⱦ��������CaO�����ʵ�����n mol��
CaO+CO2��������������ҺB�к��� Ca��OH��2 �� Na2CO3 �� �а�ɫ�������ɣ�Na2CO3+Ca��OH��2��CaCO3��+2NaOH���۽��ͳɱ�����ֹ��Ⱦ��������CaO�����ʵ�����n mol��
�����㾫����������Ҫ��������д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ�����֪ʶ�㣬��Ҫ����ע�⣺a����ƽ b������ c�����Ų�����ȷ�����⣮