��Ŀ����
 �����������⣨�û�ѧ����ʽ��ʾ����
�����������⣨�û�ѧ����ʽ��ʾ������1����һЩ�̻����������к���þ��
��2���ú���������Ļ��
��3������á�
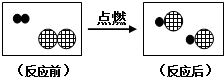
 ����ʾHԭ�ӣ��á�
����ʾHԭ�ӣ��á� ����ʾClԭ�ӣ�����ͼ�з�Ӧ�������ڵ���������
����ʾClԭ�ӣ�����ͼ�з�Ӧ�������ڵ�����������4���ڳ�ѹ��300�������£�CO2��H2��Ӧ���ɼ����ˮ��
��5���ڸ�ѹ�������¶ȡ��������������£�CO2��H2���Ը�Ч�ϳɼ��ᣨHCOOH������ʵ�ֹ�ҵ���������÷�Ӧ�Ļ�ѧ����ʽΪ
��6������ɻ����ý������ۺ�����泥�NH4ClO4���Ļ����������ȼ�ϣ���������ʹ�䱻�����������ų��������ȣ���ʹ������и���������ȷֽ⣬ͬʱ�����������壺�����ǿ����е���Ҫ�ɷ֣�һ��������������Cl2��������һ��������ˮ�����������������ƶ�������д�������漰�Ļ�ѧ��Ӧ����ʽ��
�����۱�����������������
�ڸ���������ȷֽ�
���������ȸ��ݷ�Ӧԭ���ҳ���Ӧ��������Ӧ���������ݻ�ѧ����ʽ����д���������������д���ɣ�
����⣺��1��þ��ȼ�շ���ҫ�۵İ⣬��Ӧ�Ļ�ѧ����ʽΪ��2Mg+O2
2MgO��
��2������ȼ���������������ף���Ӧ�Ļ�ѧ����ʽΪ��4P+5O2
2P2O5��
��3��ͬ�ַ��ӹ��ɵ�����Ϊ�������ͬ�ַ��ӹ��ɵ�����Ϊ������Ӧ���������������ӹ�����ͬ��Ϊͬ�����ʵķ��ӣ���ͼ����ʾ����Ϊ������ɷ�Ӧ����ʾ��ͼ��֪����Ӧ�������������������������Ȼ��⣬��Ӧ�ķ���ʽ�ǣ�H2+Cl2
2HCl��
��4���ڳ�ѹ��300�������£�CO2��H2��Ӧ���ɼ����ˮ����Ӧ�Ļ�ѧ����ʽΪ��CO2+4H2
CH4+2H2O��
��5���ڸ�ѹ�������¶ȡ��������������£�CO2��H2���Ը�Ч�ϳɼ��ᣨHCOOH������Ӧ�Ļ�ѧ����ʽΪ��CO2+H2
HCOOH��
��6�����ټ�������ʹ�䱻��������������������Ӧ�Ļ�ѧ����ʽΪ4Al+3O2
2Al2O3��
�ڸ���������ȷֽ⣬�����������壬���������ǿ����е���Ҫ�ɷ֣���������������һ��������������һ��������ˮ��������Ӧ�Ļ�ѧ����ʽΪ��2NH4ClO4
N2��+2O2��+Cl2��+4H2O����
�ʴ�Ϊ����1��2Mg+O2
2MgO����2��4P+5O2
2P2O5����3�������H2+Cl2
2HCl����4��CO2+4H2
CH4+2H2O����5��CO2+H2
HCOOH����6����4Al+3O2
2Al2O3����2NH4ClO4
N2��+2O2��+Cl2��+4H2O����
| ||
��2������ȼ���������������ף���Ӧ�Ļ�ѧ����ʽΪ��4P+5O2
| ||
��3��ͬ�ַ��ӹ��ɵ�����Ϊ�������ͬ�ַ��ӹ��ɵ�����Ϊ������Ӧ���������������ӹ�����ͬ��Ϊͬ�����ʵķ��ӣ���ͼ����ʾ����Ϊ������ɷ�Ӧ����ʾ��ͼ��֪����Ӧ�������������������������Ȼ��⣬��Ӧ�ķ���ʽ�ǣ�H2+Cl2
| ||
��4���ڳ�ѹ��300�������£�CO2��H2��Ӧ���ɼ����ˮ����Ӧ�Ļ�ѧ����ʽΪ��CO2+4H2
| ||
| 300�� |
��5���ڸ�ѹ�������¶ȡ��������������£�CO2��H2���Ը�Ч�ϳɼ��ᣨHCOOH������Ӧ�Ļ�ѧ����ʽΪ��CO2+H2
| ||
| ��ѹ�������¶� |
��6�����ټ�������ʹ�䱻��������������������Ӧ�Ļ�ѧ����ʽΪ4Al+3O2
| ||
�ڸ���������ȷֽ⣬�����������壬���������ǿ����е���Ҫ�ɷ֣���������������һ��������������һ��������ˮ��������Ӧ�Ļ�ѧ����ʽΪ��2NH4ClO4
| ||
�ʴ�Ϊ����1��2Mg+O2
| ||
| ||
| ||
| ||
| 300�� |
| ||
| ��ѹ�������¶� |
| ||
| ||
�����������ѶȲ�����ѧ�����ݷ�Ӧԭ����д��ѧ����ʽ����������ѧ����ʽ��д�������ֵĴ����в����Ͽ���ʵ�������������غ㶨�ɡ���д������������ŵȣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ