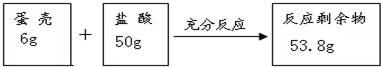
��Ŀ����
ij��ѧС��������ͼ��ʾװ�ã�ͼ�й̶�װ������ȥ���ⶨͭп�Ͻ���п������������̽���������£�
������ʵ��װ�ò����װ�������ԣ�
����װ�â��е���ƿ����2.0g��ͭп�Ͻ���Ʒ��ĩ����ע��������ע��ϡ���ᣬ������ƿ�в��ٲ�������ʱ��ȷ��ȡע�����ڶ���������10.5mLϡ���ᣬͬʱ�����н����ɼУ��Ƴ�װ�â��еĵ��ܣ�ȷ��ȡ��Ͳ��ˮ�����Ϊ214.0mL����ע����Ʒ�е����ʲ��μӷ�Ӧ
��װ�â���ʣ��������ʾ����ʵ�������ȷ����������1.4g��
�ܸ�С�����λͬѧ����ʵ����̲�ò�ͬ���ݣ�����ͭп�Ͻ���Ʒ��п������������
��ͬѧ����װ�â��з�Ӧǰ�������������м��㣻
��ͬѧ����װ�â��м���ϡ����������м��㣻
��ͬѧ����װ�â�����ȡˮ��������м��㣬����ã��ڱ�״���£��������ܶ�Ϊ0.09g/L��
�ش��������⣺
��1��װ�â��з�Ӧ�Ļ�ѧ����ʽΪ______��ʵ������������ϡ���������ٲ������壬Ŀ����______��
��2��������г���ʣ�����֮ǰ����ȷ������______��ϴ�ӡ����
��3������ʵ��ⶨ�����ݣ�������λͬѧ��______����ס������ҡ�������ͬѧ�����м�����Ʒ��п������������
��4����ѧС��ͬѧ���֣����ձ�ͬѧ�ķ������м��㣬��ʹʵ�������ֽϴ�ƫ��������ؿ������ƫ�����______�����ţ���
A���Ƴ�װ�â�ʱ��������������һ����ˮ
B��ʵ�����ǰ��û���ų�װ�â��еĿ���
C�����ݴ���ʱ��δ�ų�����ϡ������ռ�������
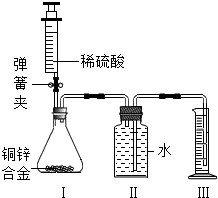
������ʵ��װ�ò����װ�������ԣ�
����װ�â��е���ƿ����2.0g��ͭп�Ͻ���Ʒ��ĩ����ע��������ע��ϡ���ᣬ������ƿ�в��ٲ�������ʱ��ȷ��ȡע�����ڶ���������10.5mLϡ���ᣬͬʱ�����н����ɼУ��Ƴ�װ�â��еĵ��ܣ�ȷ��ȡ��Ͳ��ˮ�����Ϊ214.0mL����ע����Ʒ�е����ʲ��μӷ�Ӧ
��װ�â���ʣ��������ʾ����ʵ�������ȷ����������1.4g��
�ܸ�С�����λͬѧ����ʵ����̲�ò�ͬ���ݣ�����ͭп�Ͻ���Ʒ��п������������
��ͬѧ����װ�â��з�Ӧǰ�������������м��㣻
��ͬѧ����װ�â��м���ϡ����������м��㣻
��ͬѧ����װ�â�����ȡˮ��������м��㣬����ã��ڱ�״���£��������ܶ�Ϊ0.09g/L��
�ش��������⣺
��1��װ�â��з�Ӧ�Ļ�ѧ����ʽΪ______��ʵ������������ϡ���������ٲ������壬Ŀ����______��
��2��������г���ʣ�����֮ǰ����ȷ������______��ϴ�ӡ����
��3������ʵ��ⶨ�����ݣ�������λͬѧ��______����ס������ҡ�������ͬѧ�����м�����Ʒ��п������������
��4����ѧС��ͬѧ���֣����ձ�ͬѧ�ķ������м��㣬��ʹʵ�������ֽϴ�ƫ��������ؿ������ƫ�����______�����ţ���
A���Ƴ�װ�â�ʱ��������������һ����ˮ
B��ʵ�����ǰ��û���ų�װ�â��еĿ���
C�����ݴ���ʱ��δ�ų�����ϡ������ռ�������

��1������ͭ��������ĺ��棬���������Ӧ����Ӧ����п�����ᣬ������������п��������������������������ţ�ʵ������������ϡ���������ٲ������壬Ŀ����ʹ��Ʒ�е�п��ȫ��Ӧ��
��2����ͭ������п��Һ�Ļ�����еõ�ͭ���Ƚ��й��ˣ��õ���������п��Һ��ͭ���ٽ���ϴ�ӳ�ȥ���������п���ٽ��и����ȥˮ���ɣ�
��3�����ݼ���ϡ����������м�����Ҫ֪��ϡ�������������������������û�и�֪��
��4������п�����ᷴӦ��������п������ʱ���ų����������û����ȴ�����£���������������ԭ����֪������Ͳ��ˮƫ�࣬��������Ϊ�õ��������࣬�پ������ݴ���ʱ��δ�ų�����ϡ������ռ�����������Ľ����ƫ��
�𰸣�
��1��Zn+H2SO4�TZnSO4+H2�� ʹ��Ʒ�е�п��ȫ��Ӧ��
��2�����ˣ�
��3����
��4��AC

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ