��Ŀ����
���й��ɺ��ܽ���ȫ��ȷ��һ����
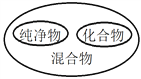
A���Խ�������ʶ | B�������ʶ����ʶ |
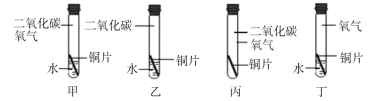
�ٿ��ô�����ҵ�������������������� �ڿ����ü�ȩˮ��Һ����ˮ��Ʒ���� ������ȱ��������ƶѪ | ��ú¯�Ϸ�һ��ˮ����Ԥ��CO�ж� ��ϴ�Ӽ�ȥ�����۵�ԭ�����黯���� ����ˮ����ԭ���ǽ��Ϳ�ȼ����Ż�� |
C������������;����ʶ | D���Ը�������� |
�������̿����ǿ�����Կ����ڷ������ ��������ѧ���ʲ����ÿ�����ʳƷ���� ����ϡ����������������Ӧ�����ڳ������������ | ���ܸı䷴Ӧ���ʵ�����һ���Ǵ��� �����κ�ˮ���ɵķ�Ӧһ�����кͷ�Ӧ �ۺϽ��������Ĵ�����ǿ�Ⱥ�Ӳ�ȸ��ߡ�����ʴ���ܸ��� |
A. A B. B C. C D. D
 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д������ҹ���������ʳ����ʷ�ƾá�������õ�һ��ȼ���ǹ���ƾ���ij��ѧ��ȤС���ͬѧ�ԡ�����ƾ��������˺��棬����ɷֽ���̽��������ش��������⡣
���������ϣ�
a������ƾ�Ҳ����Ϊ���ƾ��顰�����ȼ�Ͽ顣����ƾ������ǹ���״̬�ľƾ����ǽ��ƾ���Ӳ֬����������ư�һ���������Ȼ���Ƴɡ�
b���ƾ��Ļ�ѧʽΪC2H5OH��
c���Ȼ������Ȼ�����Һ�������ԡ�
d��BaCl2+Na2CO3��BaCO3��+2NaCl ���ɵ�BaCO3Ϊ��ɫ����
��������⣩
��1���ƾ��Ļ�ѧʽ��NaOH��ȣ����С�OH������ô�ƾ���ˮ��Һ�Dz����Լ��ԣ�
��2������ƾ��е����������Ƿ���ʼ����ʵij̶���Σ�
��ʵ��̽��1���ƾ���ˮ��Һ�Dz����Լ���
ͬѧ��ȡ�����ƾ���Һ���Թ��У��μ���ɫʯ����Һ��δ�۲쵽��ɫʯ���Ϊ��ɫ��˵���ƾ���Һ_____����ԡ����ԡ������ԡ�
��ʵ��̽��2������ƾ��е����������Ƿ���ʼ����ʵij̶����
�ٹ���ƾ��е����������Ƿ���ʡ�
ͬѧ����ȡ��������ƾ����ձ��У���������ˮ�ܽ��μ�������ϡ���ᣬ�۲쵽_____����˵�����������ѱ��ʡ���д�����������ڿ����б��ʵĻ�ѧ����ʽ_____��
��Ϊ��һ��ȷ���������Ƶı��ʳ̶ȣ��������̽����
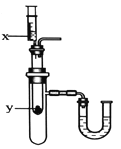
����ͬѧȡ�ձ��ϲ���Һ����֧�Թ��У�����ͼ��ʾ����ʵ�顣
ʵ�鷽�� |
|
|
ʵ������ | ��Һ��� | ����_____ |
ʵ����� | ��Һ������������ | ��Һ����̼���� |
����ͬѧ��Ϊ����ʵ�鲻��֤����Һ��һ�����������ƣ�������_____��������ȡ�ձ����ϲ���Һ���������Ȼ�����Һ����ַ�Ӧ���ã�ȡ�ϲ���Һ���μӷ�̪��Һ����̪��Һ��졣
����˼����������ʵ���м������Ȼ�����Һ��Ŀ����_____��
��ʵ�����]С��ͬѧ�������ۣ�һ����Ϊ�ù���ƾ��е��������Ʋ��ֱ��ʡ�
���ɷ���ѧϰ��ѧ����Ҫ����֮һ������ͼʾ��ȷ����
|
|
|
|
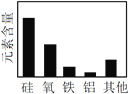
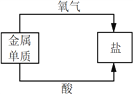
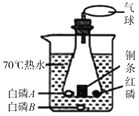
A����ѧ��Ӧ���� | B�� ���ʷ��� | C���ؿ���Ԫ�غ��� | D�������Ļ�ѧ���� |
A. A B. B C. C D. D