��Ŀ����
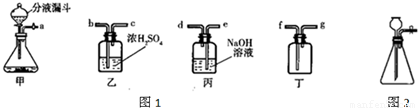
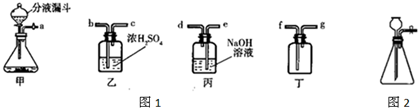
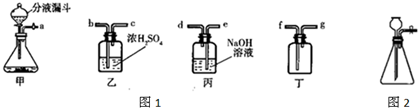
��ʵ�����п�����ͼ1װ������ȡ���壬���dz��õ����巢��װ�ã�ͨ����Һ©����������ƿ�еμ�Һ�壮��ش��������⣺
��1��ʵ����ȷ�����巢��װ��ʱӦ���ǵ�������______��
��2����ȡ����ʱ����װ�ü���ƿ�з���������̣��ڷ�Һ©����Ӧ�������______���÷�Ӧ�Ļ�ѧ����ʽΪ______��
��3����ȡ���������ʱ��װ�õ���ȷ����˳���ǣ���ܿڵ���ĸ��______��
��4��װ�ü�Ҳ��������ȡ������̼��д�����������̼ʱ��������Ӧ�Ļ�ѧ����ʽ��
��5����ͼ2װ��Ҳ�ɴ����װ����ȡ���壬��μ���װ�õ������ԣ�
��6��ȡ12gʯ��ʯ�����ʲ��μӷ�Ӧ��������ƿ�У������м���100gһ������������ϡ���ᣬ����ǡ����ȫ��Ӧ����Ӧ��������ƿ��ʣ�����ʵ�������Ϊ107.6g����ϡ���������ʵ����������Ƕ��٣�
�⣺��1��ʵ����ȷ�����巢��װ��ʱӦ���ǵ������Ƿ�Ӧ���״̬�ͷ�Ӧ������
��2���÷���װ�õ������ʺ��ù���������Һ�Ͷ���������ȡ����������ƿ�ڷ�����ǹ���������Һ����Ӧ�ķ���ʽΪ��2H2O2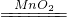 2H2O+O2����
2H2O+O2����
��3����Ũ��������������������ſ������ռ����������������ʱ����Ӧ���ǡ������̳�������װ�õ���ȷ����˳���ǣ�a��b��c��f��
��4�����������̼�ó����ʯ��ˮ����Ӧ�ķ���ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
��5����ֹˮ�м��ϣ�����©����ע��ˮ�����ڳ���©����Һ�治�½��γ�һ��ˮ����˵��װ�õ����������ã�
��6���⣺��ϡ�������Ȼ��������ΪX���������֪����Ӧ������������̼12g+100g-107.6g�T4.4g
CaCO3+2HCl�TCaCl2+H2O+CO2��
73 44
X 4.4g
 =
= X=7.3 g
X=7.3 g
ϡ���������ʵ���������= ��100%=7.3%
��100%=7.3%
��ϡ���������ʵ���������Ϊ7.3%��
�ʴ�Ϊ����1����Ӧ���״̬�ͷ�Ӧ��������2������������Һ��2H2O2 2H2O+O2������3��a��b��c��f��
2H2O+O2������3��a��b��c��f��
��4��CO2+Ca��OH��2=CaCO3��+H2O����5����ֹˮ�м��ϣ�����©����ע��ˮ��������©����Һ�治�½���˵��װ�ò�©����
��6���⣺��ϡ�������Ȼ��������ΪX���������֪����Ӧ������������̼12g+100g-107.6g�T4.4g
CaCO3+2HCl�TCaCl2+H2O+CO2��
73 44
X 4.4g
 =
= X=7.3 g
X=7.3 g
ϡ���������ʵ���������= ��100%=7.3%
��100%=7.3%
��ϡ���������ʵ���������Ϊ7.3%��
��������1������װ�õ�ѡ�����ݷ�Ӧ���״̬�ͷ�Ӧ������
��2�����ݷ���װ�õ������ʺ��ù���������Һ�Ͷ���������ȡ������
��3����������������Ũ���
��4�����������̼�ó����ʯ��ˮ�����Ƿ����ǣ�
��5������װ�������Եķ����ǣ���ֹˮ�м��ϣ�����©����ע��ˮ��������©����Һ���Ƿ��½���
��6���������ʵ�������ó�������̼��������Ȼ����ݶ�����̼����������������Ȼ�����������Ӷ���������ʵ�����������
���������⿼�����������ȡ��װ�������Եļ��鼰���ݻ�ѧ����ʽ���м��㣬����Ƚ�ȫ�棬�ؼ�Ҫ�������������е�֪ʶ���н����
��2���÷���װ�õ������ʺ��ù���������Һ�Ͷ���������ȡ����������ƿ�ڷ�����ǹ���������Һ����Ӧ�ķ���ʽΪ��2H2O2
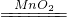 2H2O+O2����
2H2O+O2������3����Ũ��������������������ſ������ռ����������������ʱ����Ӧ���ǡ������̳�������װ�õ���ȷ����˳���ǣ�a��b��c��f��
��4�����������̼�ó����ʯ��ˮ����Ӧ�ķ���ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
��5����ֹˮ�м��ϣ�����©����ע��ˮ�����ڳ���©����Һ�治�½��γ�һ��ˮ����˵��װ�õ����������ã�
��6���⣺��ϡ�������Ȼ��������ΪX���������֪����Ӧ������������̼12g+100g-107.6g�T4.4g
CaCO3+2HCl�TCaCl2+H2O+CO2��
73 44
X 4.4g
 =
= X=7.3 g
X=7.3 gϡ���������ʵ���������=
 ��100%=7.3%
��100%=7.3%��ϡ���������ʵ���������Ϊ7.3%��
�ʴ�Ϊ����1����Ӧ���״̬�ͷ�Ӧ��������2������������Һ��2H2O2
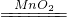 2H2O+O2������3��a��b��c��f��
2H2O+O2������3��a��b��c��f����4��CO2+Ca��OH��2=CaCO3��+H2O����5����ֹˮ�м��ϣ�����©����ע��ˮ��������©����Һ�治�½���˵��װ�ò�©����
��6���⣺��ϡ�������Ȼ��������ΪX���������֪����Ӧ������������̼12g+100g-107.6g�T4.4g
CaCO3+2HCl�TCaCl2+H2O+CO2��
73 44
X 4.4g
 =
= X=7.3 g
X=7.3 gϡ���������ʵ���������=
 ��100%=7.3%
��100%=7.3%��ϡ���������ʵ���������Ϊ7.3%��
��������1������װ�õ�ѡ�����ݷ�Ӧ���״̬�ͷ�Ӧ������
��2�����ݷ���װ�õ������ʺ��ù���������Һ�Ͷ���������ȡ������
��3����������������Ũ���
��4�����������̼�ó����ʯ��ˮ�����Ƿ����ǣ�
��5������װ�������Եķ����ǣ���ֹˮ�м��ϣ�����©����ע��ˮ��������©����Һ���Ƿ��½���
��6���������ʵ�������ó�������̼��������Ȼ����ݶ�����̼����������������Ȼ�����������Ӷ���������ʵ�����������
���������⿼�����������ȡ��װ�������Եļ��鼰���ݻ�ѧ����ʽ���м��㣬����Ƚ�ȫ�棬�ؼ�Ҫ�������������е�֪ʶ���н����

��ϰ��ϵ�д�
 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�����Ŀ
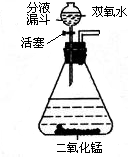
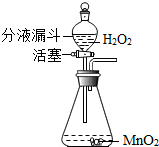 ��2006?���ţ��������⣨��ѧʽΪH2O2���׳�˫��ˮ����������һ����ɫҺ�壬����MnO2��������Ѹ�ٷֽ�ų�������ͬʱ����ˮ����ʵ�����п�����ͼ��ʾװ����˫��ˮ��ȡ������ͨ����Һ©�����������ġ��������ء�������ʱ����ƿ�еμ�˫��ˮ����
��2006?���ţ��������⣨��ѧʽΪH2O2���׳�˫��ˮ����������һ����ɫҺ�壬����MnO2��������Ѹ�ٷֽ�ų�������ͬʱ����ˮ����ʵ�����п�����ͼ��ʾװ����˫��ˮ��ȡ������ͨ����Һ©�����������ġ��������ء�������ʱ����ƿ�еμ�˫��ˮ����