��Ŀ����
����Ŀ��(5��)������ijɳ���Ҫ�����Ӫ���������˯�ߺ��ʶȵ��˶����ش��������⣺

С��ÿ���������г���ѧ������˽�ҳ���ѧ��ȣ��ô�����1�� _________________________________ (д����)�����ڲ�ע������������С�������˽����ۡ��ݹٷ�ý�屨����l6��l8����й�����������ʸߴ�70%�������۾����ܲ��ϴ�ຬͭ�����д��ѡ��ѺϽ�Ȳ��ʵġ������۾����˶�������һ����ʶ����������϶�ʱ���۾����沿Ƥ�������Ӵ��IJ��ֻ�������ͭ��[Cu2(OH)2CO3]���������۾����к��е�ͭ��������������塢ˮ�������Ϸ�Ӧ�Ľ������д���÷�Ӧ�Ļ�ѧ����ʽ��2�� ___________________________________________________����Ӧǰ��̼Ԫ�صĻ��ϼ۷ֱ�Ϊ��3�� ______________________�����ڶྵ����С��ѡ���ѻ��ѺϽ���ʵģ�����Ҫ����Ϊ����4�� ___________ (��A��B��C��ѡ��)��
A�������Ժã����ڼӹ� B���۵�� C���ܶ�С������ʴ���ܺ�
���𰸡���1����2�֣��������塢��Լ��ʯȼ�ϣ���Դ��ȼ�ϣ���������������ŷ��к����壨��Ⱦ�����˵�����������������㼴�ɣ����1���1�֣������ȫ��2�֣��ж����û�д��۷֣���2��2Cu+O2+CO2+H2O===Cu2(OH)2CO3��3��+4�ۺ�+4�ۣ�4��C
��������
��������������г���ѧ������˽�ҳ���ѧ��ȣ������ڶ������塢��Լ��ʯȼ�ϣ���Դ��ȼ�ϣ���������������ŷ��к����壨��Ⱦ���ͭ��������������塢ˮ������������ͭ�⣬����ʽΪ��2Cu+O2+CO2+H2O===Cu2(OH)2CO3�����ݻ��������������ϼ۴�����Ϊ�㣬��Ӧǰ��̼Ԫ�صĻ��ϼ۾�Ϊ+4�ۣ��Ͻ���������Ĵ�������ȣ�����ʴ���ܺá�

����Ŀ��(6��) ˮ����Һ�������������������������ʮ����Ҫ�����á�
��1����ͼʵ���У����Թ�1����������Ϊ6mLʱ���Թ�2���������ԼΪ mL��������Ӧ�Ļ�ѧ����ʽΪ________��
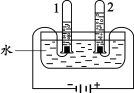
��2��ˮ���������Ƹ�����Һ���������Һ�е�����Ϊ________��
��3��ũҵ�����������ʵ���������Ϊ10% ~ 20%��NaCl��Һ��ѡ�֡��ֽ�300 g 25%��NaCl��Һϡ��Ϊ15%��NaCl��Һ����Ҫ��ˮ������Ϊ________g��
��4�������±��ش����⡣
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
�ܽ�� /g | NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 | 39.8 |
NH4Cl | 29.4 | 37.2 | 45.8 | 55.2 | 65.6 | 77.3 | |
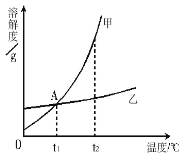
�� 60 ��ʱ���������ֱ�ʢ��50 g NaCl��NH4Cl���ձ��У�������100 g��ˮ������ܽ��Ϊ������Һ����________��Һ��
�� ������ʵ���������ձ���������40��������˵���У���ȷ����________������ĸ��ţ���
A�������ձ��е���Һ��Ϊ������Һ
B�������ձ�����Һ�����������ܲ���
C�������ձ�����Һ��������С��һ����
D�������ձ������ʵ�����������һ����С