��Ŀ����
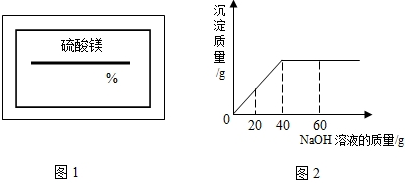
ijͬѧ��ʵ���ҷ���һƿ��ǩ��ȱ����ͼ1��ʾ������Һ��Ϊ�˲ⶨ����Һ������������������ȡ��50g����Һ����������μ���������������Ϊ10%��NaOH��Һ����Ӧ���������ɳ���������������NaOH��Һ�����Ĺ�ϵ��ͼ2��ʾ����1����֪��Ӧ�Ļ�ѧ����ʽΪ��MgSO4+2NaOH=Mg��OH��2��+Na2SO4���÷�Ӧ��������______��Ӧ��
��2������������þ��Һ�����ʵ�����������

���𰸡����������ݸ��ֽⷴӦ�Ķ��忼�ǣ�����ͼ��֪����������������Һ����Ϊ40g�������������������������þ�������ٳ�������þ��Һ�������ٷ�֮�٣�
����⣺MgSO4��NaOH���ǻ����Mg��OH��2��Na2SO4Ҳ���ǻ�����������ֻ�����������ɷ����ɵ����ֻ��������Ϊ���ֽⷴӦ��
����ͼ��֪����������������Һ����Ϊ40g�����д��������Ƶ�����Ϊ��40g×10%=4g������4g�������Ʒ�Ӧ��������þ����ΪX��
MgSO4+2NaOH=Mg��OH��2��+Na2SO4
120 80
X 4g
���ݣ� ���X=6g������þ��Һ�����ʵ���������
���X=6g������þ��Һ�����ʵ��������� =12%��
=12%��
�ʴ�Ϊ�����ֽ� 12%
�����������ؼ��Ǹ���ͼ�����������������Ƶ������������������Ƶ������������þ���������ٽ�һ����������þ������������
����⣺MgSO4��NaOH���ǻ����Mg��OH��2��Na2SO4Ҳ���ǻ�����������ֻ�����������ɷ����ɵ����ֻ��������Ϊ���ֽⷴӦ��
����ͼ��֪����������������Һ����Ϊ40g�����д��������Ƶ�����Ϊ��40g×10%=4g������4g�������Ʒ�Ӧ��������þ����ΪX��
MgSO4+2NaOH=Mg��OH��2��+Na2SO4
120 80
X 4g
���ݣ�
 ���X=6g������þ��Һ�����ʵ���������
���X=6g������þ��Һ�����ʵ��������� =12%��
=12%�� �ʴ�Ϊ�����ֽ� 12%
�����������ؼ��Ǹ���ͼ�����������������Ƶ������������������Ƶ������������þ���������ٽ�һ����������þ������������

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�
�����Ŀ
 ijͬѧ��ʵ���ҷ���һƿ��ǩ��ȱ������ͼ��ʾ������Һ��Ϊ�˲ⶨ����Һ������������������ȡ��100g����Һ���������μ�������������Ϊ10%��ϡ���ᣮ�ų��������������������ϡ�����������ϵ������ͼ��ʾ��
ijͬѧ��ʵ���ҷ���һƿ��ǩ��ȱ������ͼ��ʾ������Һ��Ϊ�˲ⶨ����Һ������������������ȡ��100g����Һ���������μ�������������Ϊ10%��ϡ���ᣮ�ų��������������������ϡ�����������ϵ������ͼ��ʾ��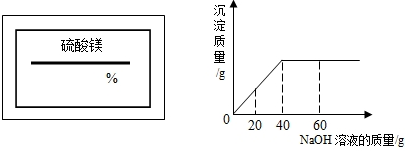
 ��2009?�Ͽ���һģ��ijͬѧ��ʵ���ҷ���һƿ��ǩ��ȱ������þ��Һ��Ϊ�˲ⶨ����Һ������������������ȡ��25g����Һ����������μ���������������Ϊ10%��NaOH��Һ����Ӧ���������ɳ���������������NaOH��Һ�����Ĺ�ϵ��ͼ��ʾ������
��2009?�Ͽ���һģ��ijͬѧ��ʵ���ҷ���һƿ��ǩ��ȱ������þ��Һ��Ϊ�˲ⶨ����Һ������������������ȡ��25g����Һ����������μ���������������Ϊ10%��NaOH��Һ����Ӧ���������ɳ���������������NaOH��Һ�����Ĺ�ϵ��ͼ��ʾ������ ��2008?��ǽ�����ijͬѧ��ʵ���ҷ���һƿ��ǩ��ȱ����ͼ��ʾ������Һ��Ϊ�˲ⶨ����Һ������������������ȡ��20g����Һ����������μ���������������Ϊ15%��MgSO4��Һ����Ӧ���������ɳ���������������MgSO4��Һ�����Ĺ�ϵ��ͼ��ʾ����������Һ����������������
��2008?��ǽ�����ijͬѧ��ʵ���ҷ���һƿ��ǩ��ȱ����ͼ��ʾ������Һ��Ϊ�˲ⶨ����Һ������������������ȡ��20g����Һ����������μ���������������Ϊ15%��MgSO4��Һ����Ӧ���������ɳ���������������MgSO4��Һ�����Ĺ�ϵ��ͼ��ʾ����������Һ����������������