��Ŀ����
2011��9�£���¶�����౻�غ����°���������������������ߵĽ��ǣ�����ͬѧ�ǿ�չ�˶�����ɷּ����õ�̽���������������ϵ�֪��

��1�����������ֹ㷺����ϴ�Ӽ���ҽ�������Ŀ�������������������������ţ����ר���ѱ�ʾ��Ŀǰ��û�п�ѧ�о�����֤�������е����������°���ʹ��Ũ�Ȳ�����0.3%���������ǰ�ȫ�ģ�������ʶȱ����ѧ�Ե���________��
A��ֹͣ�����ʹ�ø�¶���Ʒ
B��ʹ���˻�ѧ���Ӽ���ʳƷ�����嶼��Σ��
C�����ʶ������Ӱ�����������йأ����������ʱ�
D����������г��ֵ��йػ�ѧ���¼�������Ҫ�������Է�����������ȷ�ж�
��2���ܶ�Ʒ��������С����������������еġ�������ָ________��
A�������� B����Ԫ�ء�C����ԭ��
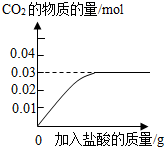
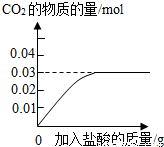
��3������ȥ����Ҫ��������Ħ�����ã�ijƷ�������е�Ħ������̼��ƣ�Ϊ�˼��鲢�ⶨ������̼��Ƶ�����������ͬѧ��ȡ��10g���࣬��������ϡ���Ტ���裮ʵ������м�¼�����������γ���ͼ���ߣ�
�ټ���10g������̼��Ƶ����ʵ���________�������ݻ�ѧ����ʽ��ʽ���㣩��
�ڸ�Ʒ��������̼��Ƶ���������Ϊ________��
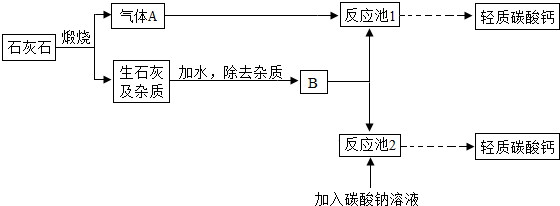
��4�������е�����̼��ƿ���ʯ��ʯ���Ʊ���ij��ѧ��ȤС�����������ת�����̣�
��֪��CaCO3+CO2+H2O��Ca��HCO3��2��Ca��HCO3��2 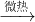 CaCO3��+CO2��+H2O
CaCO3��+CO2��+H2O
������A�Ļ�ѧʽΪ________��д����Ӧ��1�з�����Ӧ�Ļ�ѧ����ʽ________��
��Ϊ���������Ч�ʣ���ȡ����̼���ʱ��B���Ϊ________��ѡ�ʯ���顱����ʯ��ˮ������������Aͨ�뷴Ӧ��1��ʱ����öԳ������ʽ����ȣ�Ŀ���Ƿ�ֹ����________��д��ѧʽ����
�۽������ת�����̣��Աȷ�Ӧ��1�ͷ�Ӧ��2��ȡ̼��Ƶķ�����ǰ�߸�����������ɫ��ѧ�����������________��
��1��A��ֹͣ�����ʹ�ø�¶���Ʒ�����ר���ѱ�ʾ��Ŀǰ��û�п�ѧ�о�����֤�������е����������°���ʹ��Ũ�Ȳ�����0.3%���������ǰ�ȫ�ģ���A˵������ѧ��
B��ʹ���˻�ѧ���Ӽ���ʳƷ�����嶼��Σ�����������еĻ�ѧ���Ӽ����ж�����B����ѧ��
C�����ʶ������Ӱ�����������йأ����������ʱ��ǿ�ѧ�ģ�
D����������г��ֵ��йػ�ѧ���¼�������Ҫ�������Է�����������ȷ�ж��ǿ�ѧ�ģ�
�ʴ𰸣�AB��
��2����������Ԫ����ɵģ������������������еġ�������ָ��Ԫ�أ��ʴ�B��
��3�����裺̼��Ƶ����ʵ���ΪX
CaCO3+2HCl�TCaCl2+H2O+CO2��
1 1
X 0.03 mol
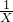 =
=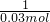
X=0.03mol
�ʴ𰸣�0.03mol
��10g��Ʒ��̼��Ƶ�����Ϊ��100��0.03mol=3g����Ʒ��̼��Ƶ���������Ϊ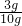 ��100%=30%
��100%=30%
�ʴ𰸣�30%��
��4������̼��Ƹ����������������ƺͶ�����̼�Ͷ�����̼���������Ʒ�Ӧ�����������Ƴ�����ˮ���ʴ𰸣�CO2��Ca��OH��2+CO2�TCaCO3��+H2O��
��ʯ���麬ˮ�������ܺͶ�����̼��Ӧ����̼��ƣ�����ʯ��ˮ������ˮ�϶�Ͷ�����̼��Ӧ����̼��ƻ�Ҫ�������������ˮ�֣�������̼���������Ƴ����·�ӦҲ������̼����ƣ�̼��������Ȳ��ȶ��ֽ�����̼��ƺ�ˮ���ʴ𰸣�ʯ���� Ca��HCO3��2��
��̼��Ʒֽ����ɵĶ�����̼���õ����������У��ʴ𰸣��������CO2��
��������1��A������ֹͣ�����ʹ�ø�¶���Ʒ�����ר���ѱ�ʾ��Ŀǰ��û�п�ѧ�о�����֤�������е����������°���ʹ��Ũ�Ȳ�����0.3%���������ǰ�ȫ�Ľ��
B������ʹ���˻�ѧ���Ӽ���ʳƷ�����嶼��Σ�����������еĻ�ѧ���Ӽ����ж����
C���������ʶ������Ӱ�����������йأ����������ʱ��ǿ�ѧ�Ľ��
D�����ö�������г��ֵ��йػ�ѧ���¼�������Ҫ�������Է�����������ȷ�ж��ǿ�ѧ�Ľ��
��2��������������Ԫ����ɵģ������������������еġ�������ָ��Ԫ�ؽ��
��3���ٸ���̼��ƺ����ᷴӦ�Ļ�ѧ����ʽ��֪̼��ƺ����ɵĶ�����̼��Ӧ��������֮��Ϊ1��1�����ͼʾ��֪������̼��������Ϊ0.03mol�����������
������̼��Ƶ���Է�����������0.03mol��Ϊ10g��Ʒ��̼��Ƶ�������Ȼ�����10g���ɽ��
��4��������̼��Ƹ����������������ƺͶ�����̼�Ͷ�����̼���������Ʒ�Ӧ�����������Ƴ�����ˮ���
������ʯ���麬ˮ�������ܺͶ�����̼��Ӧ����̼��ƣ�����ʯ��ˮ������ˮ�϶�Ͷ�����̼��Ӧ����̼��ƻ�Ҫ�������������ˮ�֣�������̼���������Ƴ����·�ӦҲ������̼����ƣ�̼��������Ȳ��ȶ��ֽ�����̼��ƺ�ˮ���
�����ý�̼��Ʒֽ����ɵĶ�����̼���õ����������н��
�����������¶�������������ѧ�Ļ�ѧ֪ʶ�����ʵ���ɡ�̼��ƺ�ϡ����ķ�Ӧ��������̼���������Ƶķ�Ӧ�����ݻ�ѧ����ʽ�ļ�������ʵĴ��Ƚ����˿��飬Ҫ����������Щ֪ʶ����д��ѧ����ʽһ��Ҫȷ����
B��ʹ���˻�ѧ���Ӽ���ʳƷ�����嶼��Σ�����������еĻ�ѧ���Ӽ����ж�����B����ѧ��
C�����ʶ������Ӱ�����������йأ����������ʱ��ǿ�ѧ�ģ�
D����������г��ֵ��йػ�ѧ���¼�������Ҫ�������Է�����������ȷ�ж��ǿ�ѧ�ģ�
�ʴ𰸣�AB��
��2����������Ԫ����ɵģ������������������еġ�������ָ��Ԫ�أ��ʴ�B��
��3�����裺̼��Ƶ����ʵ���ΪX
CaCO3+2HCl�TCaCl2+H2O+CO2��
1 1
X 0.03 mol
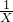 =
=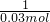
X=0.03mol
�ʴ𰸣�0.03mol
��10g��Ʒ��̼��Ƶ�����Ϊ��100��0.03mol=3g����Ʒ��̼��Ƶ���������Ϊ
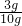 ��100%=30%
��100%=30%�ʴ𰸣�30%��
��4������̼��Ƹ����������������ƺͶ�����̼�Ͷ�����̼���������Ʒ�Ӧ�����������Ƴ�����ˮ���ʴ𰸣�CO2��Ca��OH��2+CO2�TCaCO3��+H2O��
��ʯ���麬ˮ�������ܺͶ�����̼��Ӧ����̼��ƣ�����ʯ��ˮ������ˮ�϶�Ͷ�����̼��Ӧ����̼��ƻ�Ҫ�������������ˮ�֣�������̼���������Ƴ����·�ӦҲ������̼����ƣ�̼��������Ȳ��ȶ��ֽ�����̼��ƺ�ˮ���ʴ𰸣�ʯ���� Ca��HCO3��2��
��̼��Ʒֽ����ɵĶ�����̼���õ����������У��ʴ𰸣��������CO2��
��������1��A������ֹͣ�����ʹ�ø�¶���Ʒ�����ר���ѱ�ʾ��Ŀǰ��û�п�ѧ�о�����֤�������е����������°���ʹ��Ũ�Ȳ�����0.3%���������ǰ�ȫ�Ľ��
B������ʹ���˻�ѧ���Ӽ���ʳƷ�����嶼��Σ�����������еĻ�ѧ���Ӽ����ж����
C���������ʶ������Ӱ�����������йأ����������ʱ��ǿ�ѧ�Ľ��
D�����ö�������г��ֵ��йػ�ѧ���¼�������Ҫ�������Է�����������ȷ�ж��ǿ�ѧ�Ľ��
��2��������������Ԫ����ɵģ������������������еġ�������ָ��Ԫ�ؽ��
��3���ٸ���̼��ƺ����ᷴӦ�Ļ�ѧ����ʽ��֪̼��ƺ����ɵĶ�����̼��Ӧ��������֮��Ϊ1��1�����ͼʾ��֪������̼��������Ϊ0.03mol�����������
������̼��Ƶ���Է�����������0.03mol��Ϊ10g��Ʒ��̼��Ƶ�������Ȼ�����10g���ɽ��
��4��������̼��Ƹ����������������ƺͶ�����̼�Ͷ�����̼���������Ʒ�Ӧ�����������Ƴ�����ˮ���
������ʯ���麬ˮ�������ܺͶ�����̼��Ӧ����̼��ƣ�����ʯ��ˮ������ˮ�϶�Ͷ�����̼��Ӧ����̼��ƻ�Ҫ�������������ˮ�֣�������̼���������Ƴ����·�ӦҲ������̼����ƣ�̼��������Ȳ��ȶ��ֽ�����̼��ƺ�ˮ���
�����ý�̼��Ʒֽ����ɵĶ�����̼���õ����������н��
�����������¶�������������ѧ�Ļ�ѧ֪ʶ�����ʵ���ɡ�̼��ƺ�ϡ����ķ�Ӧ��������̼���������Ƶķ�Ӧ�����ݻ�ѧ����ʽ�ļ�������ʵĴ��Ƚ����˿��飬Ҫ����������Щ֪ʶ����д��ѧ����ʽһ��Ҫȷ����

��ϰ��ϵ�д�
�����Ŀ


 Ca(HCO3)2��Ca(HCO3)2
Ca(HCO3)2��Ca(HCO3)2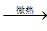
 ����CO2����H2O
����CO2����H2O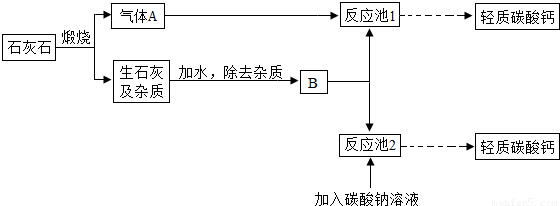
 CaCO3��+CO2��+H2O
CaCO3��+CO2��+H2O

 CaCO3��+CO2��+H2O
CaCO3��+CO2��+H2O