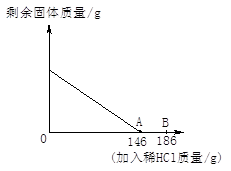��Ŀ����
(10��)̼����(Na2CO3)�׳��մ���һ�ְ�ɫ���壬�㷺���ڲ�������ֽ����֯��ϴ�Ӽ��������ȡ�������ϡ������ᷴӦ����CO2��(Na2CO3��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��Na2CO3��H2SO4===Na2SO4��CO2����H2O)�ֽ�100g Na2CO3�����ĩ(����Na2SO4����)����202gˮ�У���ȫ�ܽ⣬�ټ���100mLϡ����(�ܶ�Ϊ1.2g/mL)��ǡ����ȫ��Ӧ��ʹ����ȫ���ݳ��������ĩ�����������CO2����Ĺ�ϵ��ͼ(��״���£�CO2���ܶ�Ϊ2g/L)��ͨ�����㣺
(1)����CO2��������________________g��
(2)��100mLϡ���������ʵ������ͷ�Ӧ��������Һ����������������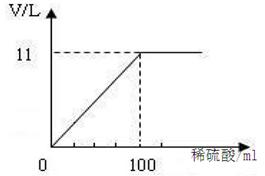
(1)����CO2��������________________g��
(2)��100mLϡ���������ʵ������ͷ�Ӧ��������Һ����������������

(1)22 (2)118g 29.5%
(1)m(CO2) ��������ܶ�=11L��2g/L��22 g
(2)��100mL������Һ��H2SO4������Ϊx��100g��Ϲ����ĩ�к�Na2CO3������Ϊy������Na2SO4������Ϊz��
Na2CO3��H2SO4��Na2SO4�� CO2����H2O
106����98������142������44
y ����x������ z������22g
������� x�� 49g
������� y�� 53g
��Ϲ����ĩ�к�Na2SO4��������100g��53g��47g
������� z�� 71g
��Ӧ��������Һ��������100g��202g��(100mL��1.2g/L)��22g�� 400g
��Ӧ���������ʵ�������47g��71g��118g
��Ӧ��������Һ��������������100%��29.5%
�𣺷�Ӧ���������ʵ�����Ϊ118g, ��Ӧ��������Һ����������Ϊ29.5%��
(2)��100mL������Һ��H2SO4������Ϊx��100g��Ϲ����ĩ�к�Na2CO3������Ϊy������Na2SO4������Ϊz��
Na2CO3��H2SO4��Na2SO4�� CO2����H2O
106����98������142������44
y ����x������ z������22g
������� x�� 49g
������� y�� 53g
��Ϲ����ĩ�к�Na2SO4��������100g��53g��47g
������� z�� 71g
��Ӧ��������Һ��������100g��202g��(100mL��1.2g/L)��22g�� 400g
��Ӧ���������ʵ�������47g��71g��118g
��Ӧ��������Һ��������������100%��29.5%
�𣺷�Ӧ���������ʵ�����Ϊ118g, ��Ӧ��������Һ����������Ϊ29.5%��

��ϰ��ϵ�д�
 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����Ŀ
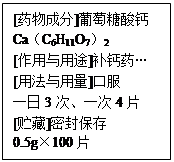

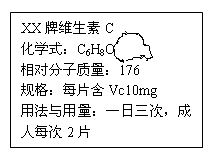
 2CuO + H2O +
2CuO + H2O + ������
������