��Ŀ����
�����������������о��й㷺��Ӧ�á�
��1�����н�����Ʒ�У����ý��������Ե���_________������ĸ��ţ���
A���ƽ���Ʒ B������ C��������
��2��Ϊ�˱������Ľ�����Դ������������������__________
A�������������Ϲ�
B��������Ʒ����ˢ�ᡢͿ�ͣ���ֹ����
C����������̽��������ȫ������
D�������з��²����������������
��3��ijʵ���ҵķ�Һ�к���CuSO4��FeSO4��H2SO4��Ϊ�������������ͽ���ͭ�����������ͼ��ʾ���̣�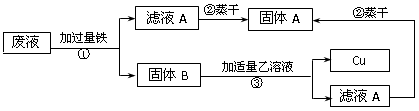
����ٵIJ�����__________������ٷ����Ļ�ѧ��Ӧ����ʽΪ____________��������з����Ļ�ѧ��Ӧ�Ļ�������Ϊ____________��
(1)C
(2)ABD
(3)���� Fe+ CuSO4 = FeSO4 + Cu Fe+ H2SO4 = FeSO4 + H2�� �û���Ӧ
���������������1�����������������ý��������ԣ�
��2������������Դ�Ĵ�ʩ�У���ֹ������ʴ���������ã��������ɽ�����Դ��Ѱ�ҽ��������Ʒ��
��3���������������ͽ���ͭ ����Ҫ�����ᷴӦ�����������û���Ӧ��CuSO4��Һ�е�ͭ�û���������������ͼ������һ�ѹ����Һ������ˣ����ǹ��˵IJ��裻�����������������ᷴӦ��Ҳ�ܰ�����ͭ��Һ�е�ͭ�û�������Fe+ CuSO4 = FeSO4 + Cu �� Fe+ H2SO4 = FeSO4 + H2�� �����ڼ�������ǹ����ģ��ʹ���B������ͭ�Ļ�����ҺA��������������������Һ��������Һ�������������������û���Ӧ��
���㣺���������˲������û���Ӧ

 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д���ѧ������������
��1�������Ǹ����Ͷ��ĺ��ӣ�ƽʱ�Լ������������Լ���ϴ����������ʪ��ȡ����ϴ�·�ʱ�о����ַ��̣�ԭ����ϴ�·�����ˮ ������ȡ������ȡ�����������ˮ�ھ��������г��� ����ɫ�غ���ζ��һ�����ͨ�� �ķ�������ˮ��Ӳ�ȡ�
��2��������ʵ����ͨ���þƾ��Ƹ����ʼ��ȣ���ȼ�յ�ȼ�����Ҵ��������ƾ����ƾ���һ�������Դ�������� ��ѡ������ �� ���������� ��ѡ�����������������������Դ���ƾ�ȼ�յĻ�ѧ����ʽΪ ��
��3��PM2.5��ָ������ֱ��С�ڻ����2.5�Ŀ����Ҳ��Ϊ����ο��������ֱ���������˵�ͷ��˿��ϸ��1/20����ϴֵĴ�����������ȣ�PM2.5����С�������������ж����к��������ڴ����е�ͣ��ʱ�䳤�����;���Զ����������彡���ʹ�������������Ӱ���������������������֧��������Ѫ�ܲ��ȷ���ļ�������ն�ȥҰ����ɭ�ֻߣ�����һ�ߣ������PM2.5ֵ�ܵͣ�
��PM2.5��ʵ�����ҹ����������Ҫ��Ⱦ��� ������ĸ��ţ���
| A���������� | B���������� | C��һ����̼ | D������������� |
�����ճ������У����Լ���PM2.5��һ����Ч��ʩ��
��8�֣�ij��ѧ��ȤС���ijƷ��������Ħ�����ɷּ��京������̽���������������£�
1��������Ħ������̼��ơ�����������ɣ�
2�������������ɷ������������������ɣ�
3����ʯ�ҵ���Ҫ�ɷ�Ϊ��ʯ�Һ��������ƹ��塣
������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3��������������ȷ��̼��Ƶ�������������ش��������⣺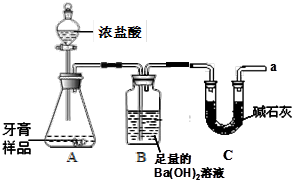
��1��B�з�Ӧ����BaCO3�Ļ�ѧ����ʽ����������������������������������������
��2��Cװ�õ�������������������������������������������
��3�����и����ʩ�У�������߲ⶨȷ�ȵ���������������������ţ���
| A���ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2���� |
| B���μ�����˹��� |
| C����A~B֮������ʢ��Ũ�����ϴ��װ�� |
| D����A~B֮������ʢ�б���̼��������Һ��ϴ��װ�� |
����������������������������� �������������� ��
��5��ijͬѧ��Ϊ���زⶨB�����ɵ�BaCO3��������B�е�Ba��OH��2��Һ����ŨH2SO4 ��ͨ���ⶨCװ�÷�Ӧǰ���������Ҳ���ԲⶨCaCO3������������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ������������������������������������������������
��6��ʵ����ȷ��ȡ8.00g��Ʒ���ݣ��������βⶨ�����BaCO3ƽ������Ϊ3.94g ���������Ʒ��̼��Ƶ�������������д��������̣�
��ҵ�ϳ���ú������ʹ����ȼ�գ���Ŀ����
| A������ú���˷� | B���������������� |
| C�����ٶ�����̼���ŷ� | D������������γ� |
�����仯��������������������Ҫ��Ӧ�á���ش��������⣺
��1����ʢ�������ļ���ƿ�е�ȼϸ��˿��������ȼ�յĻ�ѧ����ʽ��___________________________________��Ϊ��ֹ����ƿ���ѣ�����ȡ�Ĵ�ʩ��__________________________________________________________________��
��2����֪�������Ȼ�����Ӧ�����Ȼ���������������������������Ҫ�ɷ���Fe2O3�����������У���ַ�Ӧ������ʣ�࣬д�������û���Ӧ�Ļ�ѧ����ʽ_____________________________________����Һ�еĽ�����������____________���÷��ű�ʾ����
��3����¯�����У���̿��������_______________________________________________________________���û�ѧ����ʽ��ʾ����
��4�������ۺ�ͭ�۵Ļ���������������Һ�У���Ӧ�������й���ʣ�ࡣ����˵����ȷ����____________����д��ĸ��ţ���
| A��ʣ�����϶������� | B��ʣ�����϶�������ͭ |
| C����Ӧ����Һ��һ����Fe2+��Cu2+ | D����Ӧ����Һ�п��ܺ���Ag+��Cu2+ |
�г��ϡ����ȷ��С��еĻ�ѧ��Ӧ�ǣ�Mg+2H2O �� Mg(OH)2+H2����������������
| A��Mg | B��H2O | C��Mg(OH)2 | D��H2 |
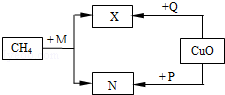

 CO2��Fe2O3��3H2
CO2��Fe2O3��3H2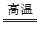 2Fe��3H2O
2Fe��3H2O