ΧβΡΩΡΎ»ί
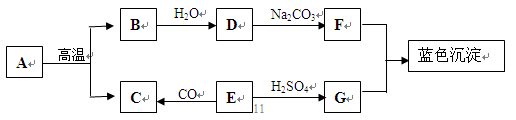
Θ®1Θ©»γΆΦΥυ ΨΘ§Ρ≥ΜλΚœΤχΧεΚ§”–≥θ÷–Μ·―ß≥ΘΦϊΒΡΤχΧεΘ§ΫΪΤδ“ά¥ΈΆ®ΙΐΉΑ”–≥Έ«εΒΡ ·Μ“Υ°ΒΡΦΉ““ΝΫΗω ‘ΦΝΤΩΘ§ΦΉΈόΟςœ‘œ÷œσΘ§““÷–ΒΡ≥Έ«εΒΡ ·Μ“Υ°±δΜκΉ«ΓΘ‘ρΗΟΤχΧεΒΡ≥…Ζ÷ΈΣ___Γχ___ΘΜΦΉ÷–ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ___Γχ___ΓΘ
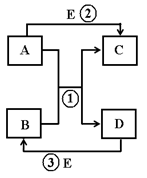
Θ®2Θ©œ÷”–œΓ―ΈΥαΓΔ ·Μ“Υ°ΓΔ ≥―ΈΥ°ΓΔΧΦΥαΡΤ»ή“ΚΥΡ÷÷Έό…Ϊ»ή“ΚΘ§ΈΣΦχ±πΥϋΟ«Ζ÷±π±ύΚ≈ΈΣAΓΔBΓΔCΓΔDΘ§»ΜΚσΗς»Γ…Ό–μΫχ––ΝΫΝΫΜλΚœ Β―ιΘ§œ÷œσΦϊ”“±μΘ®ΆΦ÷–ΓΑΓΐΓ±ΓΑΓϋΓ±ΓΑΘ≠Γ±ΖϊΚ≈Ζ÷±π±μ Ψ…ζ≥…≥ΝΒμΓΔΤχΧεΚΆΈόΟςœ‘±δΜ·Θ©ΓΘΗυΨί±μ÷– Β―ιœ÷œσ≈–Εœ
ΔΌBΈΣ___Γχ___Θ®ΧνΜ·―ß ΫΘ©ΘΜ
ΔΎA”κCΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ___Γχ___Θ§ΗΟΖ¥”ΠΒΡΜυ±ΨΖ¥”Πάύ–Ά «___Γχ___ΓΘ
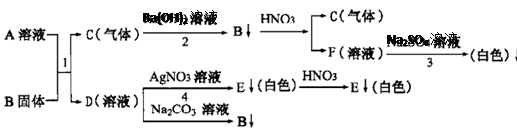
Θ®3Θ©Ρ≥ΩΈΆβ―ßœΑ–ΓΉι”ΟΧΦΥαΡΤ»ή“ΚΖ÷±πΦχ±π≥Έ«ε ·Μ“Υ°”κ―ΈΥαΓΘ Β―ιΚσΫΪΖœ“ΚΒΙ»κΆ§“Μ…’±≠÷–Θ§ΉνΚσ…’±≠ΡΎ“ΚΧε≥Έ«εΓΘάœ Π“ΐΒΦΩΈΆβ–ΓΉιΒΡΆ§―ßΧ÷¬έΖ÷ΈωΚσ»œΈΣΘ§…’±≠÷–≥Έ«εΒΡ»ή“ΚΩ…ΡήΜαΚ§”–œ¬Ν–Έο÷ ΘΚNa2CO3ΓΔHClΓΔCa(OH)2ΓΔNaOHΓΔNaClΓΔCaCl2Θ§«κΡψΗυΨί≥θ»ΐΜ·―ßΥυ―ß÷Σ Ε≈–ΕœΘΚΗΟ…’±≠≥Έ«ε»ή“Κ÷–“ΜΕ®Κ§”–ΒΡ»ή÷ «___Γχ___Θ§“ΜΕ®≤ΜΚ§”–ΒΡ»ή÷ «___Γχ___ΓΘ

| | A | B | C | D |
| A | Θ≠ | Θ≠ | Γΐ | Θ≠ |
| B | Θ≠ | Θ≠ | Γϋ | Θ≠ |
| C | Γΐ | Γϋ | ΓΣ | Θ≠ |
| D | Θ≠ | Θ≠ | Θ≠ | Θ≠ |
ΔΌBΈΣ___Γχ___Θ®ΧνΜ·―ß ΫΘ©ΘΜ
ΔΎA”κCΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ___Γχ___Θ§ΗΟΖ¥”ΠΒΡΜυ±ΨΖ¥”Πάύ–Ά «___Γχ___ΓΘ
Θ®3Θ©Ρ≥ΩΈΆβ―ßœΑ–ΓΉι”ΟΧΦΥαΡΤ»ή“ΚΖ÷±πΦχ±π≥Έ«ε ·Μ“Υ°”κ―ΈΥαΓΘ Β―ιΚσΫΪΖœ“ΚΒΙ»κΆ§“Μ…’±≠÷–Θ§ΉνΚσ…’±≠ΡΎ“ΚΧε≥Έ«εΓΘάœ Π“ΐΒΦΩΈΆβ–ΓΉιΒΡΆ§―ßΧ÷¬έΖ÷ΈωΚσ»œΈΣΘ§…’±≠÷–≥Έ«εΒΡ»ή“ΚΩ…ΡήΜαΚ§”–œ¬Ν–Έο÷ ΘΚNa2CO3ΓΔHClΓΔCa(OH)2ΓΔNaOHΓΔNaClΓΔCaCl2Θ§«κΡψΗυΨί≥θ»ΐΜ·―ßΥυ―ß÷Σ Ε≈–ΕœΘΚΗΟ…’±≠≥Έ«ε»ή“Κ÷–“ΜΕ®Κ§”–ΒΡ»ή÷ «___Γχ___Θ§“ΜΕ®≤ΜΚ§”–ΒΡ»ή÷ «___Γχ___ΓΘ
Θ®1Θ©__HClΚΆCO2__Θ§ Ca(OH)2+2HCl=CaCl2+2H2O ΓΘ
Θ®2Θ©ΔΌ HCl ΘΜΔΎ Na2CO3+Ca(OH)2=CaCO3Γΐ+2NaOH Θ§Η¥Ζ÷ΫβΖ¥”Π ΓΘ
Θ®3Θ© NaClΓΔCaCl2 Θ§ Ca(OH)2ΓΔNa2CO3ΓΔNaOH ΓΘ
Θ®2Θ©ΔΌ HCl ΘΜΔΎ Na2CO3+Ca(OH)2=CaCO3Γΐ+2NaOH Θ§Η¥Ζ÷ΫβΖ¥”Π ΓΘ
Θ®3Θ© NaClΓΔCaCl2 Θ§ Ca(OH)2ΓΔNa2CO3ΓΔNaOH ΓΘ
Ζ÷ΈωΘΚΘ®1Θ©ΗυΨίΦΉΈόΟςœ‘œ÷œσΘ§““÷–ΒΡ≥Έ«εΒΡ ·Μ“Υ°±δΜκΉ«Θ°≈–ΕœΜλΚœΤχΧεΒΡ≥…Ζ÷ΘΜ
Θ®2Θ©ΗυΨί±μΗώ÷–A”κCΖ¥”Π…ζ≥…≥ΝΒμΘ§»ΖΕ®AΓΔC « ·Μ“Υ°ΜρΧΦΥαΡΤ»ή“ΚΘΜ”÷“ρB”κCΖ¥”Π…ζ≥…ΤχΧεΘ§C «ΧΦΥαΡΤ»ή“ΚΘ§B «œΓ―ΈΥαΘΜ»ΖΕ®ΗςΈο÷ Κσ–¥≥ωA”κCΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘ§“‘ΦΑΖ¥”Πάύ–ΆΘΜ
Θ®3Θ©ΗυΨίΝΫ÷÷Ζ¥”ΠΒΡΖ¥”ΠΈοΓΔ…ζ≥…Έο“‘ΦΑΥϊΟ«÷°ΦδΒΡΖ¥”Π≈–ΕœΘ°
Ϋβ¥πΘΚΫβΘΚΘ®1Θ©ΜλΚœΤχΧεΒΡ≥…Ζ÷ «HClΚΆCO2Θ§÷ς“Σ «¬»Μ·«β÷–ΚΆΝΥ«β―θΜ·ΗΤΘ§ΒΦ÷¬Εΰ―θΜ·ΧΦΈόΖ®”κ«β―θΜ·ΗΤΖ¥”Π…ζ≥…≥ΝΒμΘΜΥυ“‘ΦΉΈόΟςœ‘œ÷œσΘ§““÷–ΒΡ≥Έ«εΒΡ ·Μ“Υ°±δΜκΉ«Θ°ΦΉ÷–ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚCaΘ®OHΘ©2+2HCl=CaCl2+2H2OΘ°
Ι ¥πΑΗΈΣΘΚHClΚΆCO2ΘΜCaΘ®OHΘ©2+2HCl=CaCl2+2H2OΘ°
Θ®2Θ©ΔΌA”κCΖ¥”Π…ζ≥…≥ΝΒμΘ§»ΖΕ®AΓΔC « ·Μ“Υ°ΜρΧΦΥαΡΤ»ή“ΚΘΜ”÷“ρB”κCΖ¥”Π…ζ≥…ΤχΧεΘ§C «ΧΦΥαΡΤ»ή“ΚΘ§B «œΓ―ΈΥαΘΜA « ·Μ“Υ°ΘΜD « ≥―ΈΥ°Θ°
Ι ¥πΑΗΈΣΘΚHClΘΜ
ΔΎΗυΨίA « ·Μ“Υ°Θ§C «ΧΦΥαΡΤ»ή“ΚΘ§Εΰ’ΏΖ¥”ΠΒΡΜ·―ß Ϋ «ΘΚNa2CO3+CaΘ®OHΘ©2=CaCO3Γΐ+2NaOHΘΜ «ΝΫ÷÷Μ·ΚœΈοœύΜΞΫΜΜΜ≥…Ζ÷Θ§…ζ≥…ΝμΆβΝΫ÷÷Μ·ΚœΈοΒΡΗ¥Ζ÷ΫβΖ¥”ΠΘ°
Ι ¥πΑΗΈΣΘΚNa2CO3+CaΘ®OHΘ©2=CaCO3Γΐ+2NaOHΘΜΗ¥Ζ÷ΫβΖ¥”ΠΘΜ
Θ®3Θ©ΗυΨίΧΦΥαΡΤ»ή“Κ”κ ·Μ“Υ°ΒΡΜ·―ßΖ¥”Π Ϋ «ΘΚNa2CO3+CaΘ®OHΘ©2®T2NaOH+CaCO3ΓΐΘΜΧΦΥαΡΤ»ή“Κ”κœΓ―ΈΥαΒΡΜ·―ßΖ¥”Π Ϋ «ΘΚNa2CO3+2HCl®T2NaCl+H2O+CO2ΓϋΘ°Na2CO3ΓΔHClΓΔCaΘ®OHΘ©2ΓΔNaOHΓΔNaClΓΔCaCl2÷–NaCl «…ζ≥…ΈοΘ§≤Δ«“≤Μ”κΤδΥϊΈο÷ Ζ¥”ΠΘ§Υυ“‘“ΜΕ®”–ΘΜ Β―ιΚσΫΪΖœ“ΚΒΙ»κΆ§“Μ…’±≠÷–Θ§ΉνΚσ…’±≠ΡΎ“ΚΧε≥Έ«εΘ°ΥΒΟς…ζ≥…ΒΡ≥ΝΒμCaCO3”κΕύ”ύΒΡ―ΈΥαΖ¥”ΠΝΥΘ§≤Δ…ζ≥…ΝΥCaCl2Θ§≤Δ≤Μ‘Ό”κΤδΥϊΈο÷ Ζ¥”ΠΘ§Υυ“‘“≤“ΜΕ®”–ΘΜCaΘ®OHΘ©2ΓΔNa2CO3ΓΔNaOHΕΦΡή”κ―ΈΥαΖ¥”ΠΘ§Υυ“‘“ΜΕ®ΟΜ”–Θ°
Ι ¥πΑΗΈΣΘΚNaClΓΔCaCl2ΘΜCaΘ®OHΘ©2ΓΔNa2CO3ΓΔNaOHΘ°
Θ®2Θ©ΗυΨί±μΗώ÷–A”κCΖ¥”Π…ζ≥…≥ΝΒμΘ§»ΖΕ®AΓΔC « ·Μ“Υ°ΜρΧΦΥαΡΤ»ή“ΚΘΜ”÷“ρB”κCΖ¥”Π…ζ≥…ΤχΧεΘ§C «ΧΦΥαΡΤ»ή“ΚΘ§B «œΓ―ΈΥαΘΜ»ΖΕ®ΗςΈο÷ Κσ–¥≥ωA”κCΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘ§“‘ΦΑΖ¥”Πάύ–ΆΘΜ
Θ®3Θ©ΗυΨίΝΫ÷÷Ζ¥”ΠΒΡΖ¥”ΠΈοΓΔ…ζ≥…Έο“‘ΦΑΥϊΟ«÷°ΦδΒΡΖ¥”Π≈–ΕœΘ°
Ϋβ¥πΘΚΫβΘΚΘ®1Θ©ΜλΚœΤχΧεΒΡ≥…Ζ÷ «HClΚΆCO2Θ§÷ς“Σ «¬»Μ·«β÷–ΚΆΝΥ«β―θΜ·ΗΤΘ§ΒΦ÷¬Εΰ―θΜ·ΧΦΈόΖ®”κ«β―θΜ·ΗΤΖ¥”Π…ζ≥…≥ΝΒμΘΜΥυ“‘ΦΉΈόΟςœ‘œ÷œσΘ§““÷–ΒΡ≥Έ«εΒΡ ·Μ“Υ°±δΜκΉ«Θ°ΦΉ÷–ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚCaΘ®OHΘ©2+2HCl=CaCl2+2H2OΘ°
Ι ¥πΑΗΈΣΘΚHClΚΆCO2ΘΜCaΘ®OHΘ©2+2HCl=CaCl2+2H2OΘ°
Θ®2Θ©ΔΌA”κCΖ¥”Π…ζ≥…≥ΝΒμΘ§»ΖΕ®AΓΔC « ·Μ“Υ°ΜρΧΦΥαΡΤ»ή“ΚΘΜ”÷“ρB”κCΖ¥”Π…ζ≥…ΤχΧεΘ§C «ΧΦΥαΡΤ»ή“ΚΘ§B «œΓ―ΈΥαΘΜA « ·Μ“Υ°ΘΜD « ≥―ΈΥ°Θ°
Ι ¥πΑΗΈΣΘΚHClΘΜ
ΔΎΗυΨίA « ·Μ“Υ°Θ§C «ΧΦΥαΡΤ»ή“ΚΘ§Εΰ’ΏΖ¥”ΠΒΡΜ·―ß Ϋ «ΘΚNa2CO3+CaΘ®OHΘ©2=CaCO3Γΐ+2NaOHΘΜ «ΝΫ÷÷Μ·ΚœΈοœύΜΞΫΜΜΜ≥…Ζ÷Θ§…ζ≥…ΝμΆβΝΫ÷÷Μ·ΚœΈοΒΡΗ¥Ζ÷ΫβΖ¥”ΠΘ°
Ι ¥πΑΗΈΣΘΚNa2CO3+CaΘ®OHΘ©2=CaCO3Γΐ+2NaOHΘΜΗ¥Ζ÷ΫβΖ¥”ΠΘΜ
Θ®3Θ©ΗυΨίΧΦΥαΡΤ»ή“Κ”κ ·Μ“Υ°ΒΡΜ·―ßΖ¥”Π Ϋ «ΘΚNa2CO3+CaΘ®OHΘ©2®T2NaOH+CaCO3ΓΐΘΜΧΦΥαΡΤ»ή“Κ”κœΓ―ΈΥαΒΡΜ·―ßΖ¥”Π Ϋ «ΘΚNa2CO3+2HCl®T2NaCl+H2O+CO2ΓϋΘ°Na2CO3ΓΔHClΓΔCaΘ®OHΘ©2ΓΔNaOHΓΔNaClΓΔCaCl2÷–NaCl «…ζ≥…ΈοΘ§≤Δ«“≤Μ”κΤδΥϊΈο÷ Ζ¥”ΠΘ§Υυ“‘“ΜΕ®”–ΘΜ Β―ιΚσΫΪΖœ“ΚΒΙ»κΆ§“Μ…’±≠÷–Θ§ΉνΚσ…’±≠ΡΎ“ΚΧε≥Έ«εΘ°ΥΒΟς…ζ≥…ΒΡ≥ΝΒμCaCO3”κΕύ”ύΒΡ―ΈΥαΖ¥”ΠΝΥΘ§≤Δ…ζ≥…ΝΥCaCl2Θ§≤Δ≤Μ‘Ό”κΤδΥϊΈο÷ Ζ¥”ΠΘ§Υυ“‘“≤“ΜΕ®”–ΘΜCaΘ®OHΘ©2ΓΔNa2CO3ΓΔNaOHΕΦΡή”κ―ΈΥαΖ¥”ΠΘ§Υυ“‘“ΜΕ®ΟΜ”–Θ°
Ι ¥πΑΗΈΣΘΚNaClΓΔCaCl2ΘΜCaΘ®OHΘ©2ΓΔNa2CO3ΓΔNaOHΘ°

ΝΖœΑ≤αœΒΝ–¥πΑΗ
 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
œύΙΊΧβΡΩ