��Ŀ����
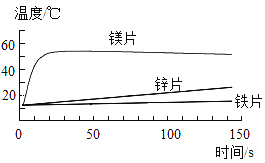
����Ŀ��A��B��C��D���Ǿ��꼶��ѧ�̲��нϳ��������ʣ����Ǵ�����ͼ��ʾ��ת����ϵ����Ӧ������ȥ����
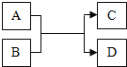
��1����AΪ����Һ��CΪ���嵥�ʣ���÷�Ӧ�Ļ�����Ӧ������____��A��Һ��һ�����е�������_____��д��ѧ���ţ���
��2���ֱ�д��һ����������Ҫ��Ļ�ѧ����ʽ��
����A��C��Ϊ�������ʣ�________��
����A������л��CΪ������ܼ���________��
��3����AΪ��ɫ��Һ����Ӧ�г�����ɫ��������д���ó����Ļ�ѧʽ________��
���𰸡��û���Ӧ H+ CuSO4+ Fe= FeSO4 + Cu���������ɣ� CH4+ 2O2![]() CO2+ 2H2O Cu��OH��2
CO2+ 2H2O Cu��OH��2
��������
��1����AΪ����Һ��CΪ���嵥�ʣ��ϻ��ý������ᷴӦ�����κ������������û���Ӧ����Һ�����������ӣ�
��2������A��CΪ�������ʣ�B��D���ǻ�������ǽ���������Һ�����û���Ӧ��������Ӧ�Ļ�ѧ����ʽΪFe+CuSO4=Cu+FeSO4��
����A������л��CΪˮ��AΪ���飬����������ڵ�ȼ�����������ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽ��CH4+ 2O2![]() CO2+ 2H2O��
CO2+ 2H2O��
��3����AΪ��ɫ��Һ�����ɵ�C����ɫ�����������������ͭ���������Ʒ�Ӧ����������ͭ�����������ƣ�������Ӧ�Ļ�ѧ����ʽΪCuSO4+2NaOH=Na2SO4+Cu��OH��2���������Ļ�ѧʽΪ��Cu��OH��2��

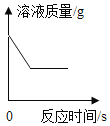
����Ŀ����һ�ܱ������ڼ���ס��ҡ��������������ʣ���÷�Ӧǰ������ʵ��������±���ʾ��
���� | �� | �� | �� | �� |
��Ӧǰ����/g | 50 | 1 | 1 | 23 |
��Ӧ������/g | 2 | 45 | 28 | x |
����˵��������ǣ�������
A.x����0
B.�÷�Ӧ����Ϊ�û���Ӧ
C.�÷�Ӧ�м����������ı仯��Ϊ48�� 23
D.�÷�Ӧ��ѭ�����غ㶨��
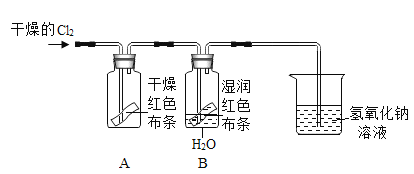
����Ŀ��ijУ����ѧ��ȥ����ɽ���Σ������˼���ʯ��ʯ��Ʒ��Ϊ�˼����Ʒ��̼��Ƶĺ������ס��ҡ���������λͬѧ������������ͬ����������Ʒ��ַ�Ӧ�����вⶨ![]() ������Ʒ�е����ʲ�����ˮ���Ҳ������ᷴӦ
������Ʒ�е����ʲ�����ˮ���Ҳ������ᷴӦ![]() ��������������
��������������
�� | �� | �� | �� | |
������ĥ���ʯ��ʯ��Ʒ���� |
|
|
|
|
��������� |
|
|
|
|
ʣ���������� |
|
|
|
|
���ʴ�
��1��![]() ��Ʒ��
��Ʒ��![]() �����ַ�Ӧ�������Ƿ���ʣ��______
�����ַ�Ӧ�������Ƿ���ʣ��______ ![]() ����������������
����������������![]() ����Ʒ��̼��Ƶ�����������______��
����Ʒ��̼��Ƶ�����������______��
��2�����������������������![]() ��������
��������![]()
��3������![]() ��Ʒ��
��Ʒ��![]() �����Ϻ����������̼������������Ĺ�ϵͼ��
�����Ϻ����������̼������������Ĺ�ϵͼ��

����Ŀ�����и������ʼ���ͨ��һ����Ӧ����ʵ����ͼת�����ǣ�������
X | Y | Z | |
A | Fe | FeCl2 | Fe2O3 |
B | O2 | CuO | Cu |
C | AgNO3 | Ba��NO3��2 | BaSO4 |
D | Ca��OH��2 | NaOH | H2O |

A. AB. BC. CD. D