��Ŀ����
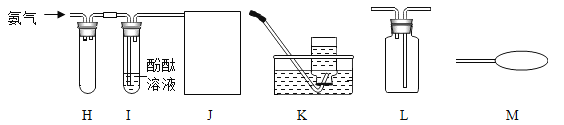
����Ŀ��ijУ����ѧ��ȥ����ɽ���Σ������˼���ʯ��ʯ��Ʒ��Ϊ�˼����Ʒ��̼��Ƶĺ������ס��ҡ���������λͬѧ������������ͬ����������Ʒ��ַ�Ӧ�����вⶨ![]() ������Ʒ�е����ʲ�����ˮ���Ҳ������ᷴӦ
������Ʒ�е����ʲ�����ˮ���Ҳ������ᷴӦ![]() ��������������
��������������
�� | �� | �� | �� | |
������ĥ���ʯ��ʯ��Ʒ���� |
|
|
|
|
��������� |
|
|
|
|
ʣ���������� |
|
|
|
|
���ʴ�
��1��![]() ��Ʒ��
��Ʒ��![]() �����ַ�Ӧ�������Ƿ���ʣ��______
�����ַ�Ӧ�������Ƿ���ʣ��______ ![]() ����������������
����������������![]() ����Ʒ��̼��Ƶ�����������______��
����Ʒ��̼��Ƶ�����������______��
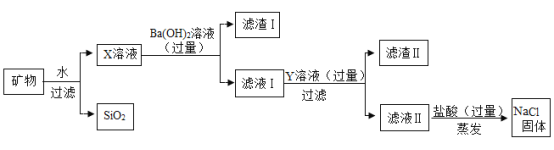
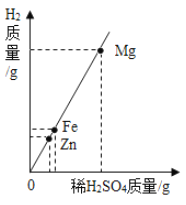
��2�����������������������![]() ��������
��������![]()
��3������![]() ��Ʒ��
��Ʒ��![]() �����Ϻ����������̼������������Ĺ�ϵͼ��
�����Ϻ����������̼������������Ĺ�ϵͼ��

���𰸡���1����90%
��2��14.6%
��3��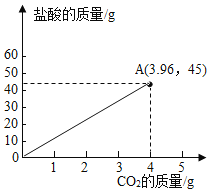
��������
��1���ɱ��е����ݿ�֪��ÿ10g��ϡ�����ܷ�Ӧ��̼��Ƶ�����Ϊ2g�����ʵ�����Ϊ1g����9g̼�����ȫ��Ӧ��Ҫ45.0g���ᣬ��10.0g��Ʒ��45.0g�����ַ�Ӧ������û��ʣ�ࣻ
�ɶ������ݿ�֪�����ʵ�����Ϊ1.0g��10.0g��Ʒ��̼��Ƶ�����Ϊ��![]() ��̼������������ǣ�
��̼������������ǣ�![]() ��
��
��2����45.0gϡ���������ʵ�����Ϊx�����ɶ�����̼������Ϊy
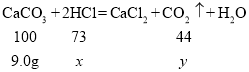
![]()
![]()
![]() y=3.96g
y=3.96g
�������������������![]()
���������������������![]() ��
��
��3��������̼��Ʒ�Ӧ�����Ȼ��ơ�������̼��ˮ�����ŷ�Ӧ�Ľ��У�������̼�����������ӣ����������ݿ�֪��10.0g��Ʒ��45.0g����ǡ����ȫ��Ӧ�����ɶ�����̼������Ϊ3.96g����10.0g��Ʒ��50.0g�����Ϻ����������̼������������Ĺ�ϵͼΪ��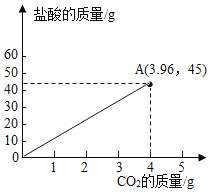

����Ŀ��2020�����ͻ������������״������Խ��Խ�������ʶ���ճ������������Ҫ�ԡ�ҽ�þƾ���84����Һ��Ϊÿ����ͥ�ر���������Ʒ��
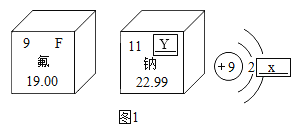
����������һ�����ھƾ���
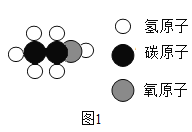
(1)��ͼ�Ǿƾ�(C2H5OH)���ӵ���ģ��ͼ1���ƾ���ѧ����_____������_____(�����)��
A ������ B ������ C ���� D ���
(2)�ƾ�����ȼ�ϣ�ȼ��ʱ�ų��������ȣ��仯ѧ��Ӧ����ʽΪ_____��ʹ�ƾ��߱���ȼ����һ���ʵ�����_____(��������)��Ϩ��ƾ���ʱ�õ�ñ�������ݵ����ԭ����_____��
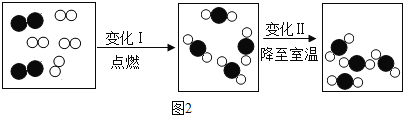
(3)�ƾ��ǿ�������Դ������ũ����ո������ƾ�����Ҫ������ͼ2��ʾ����ش��������⣺
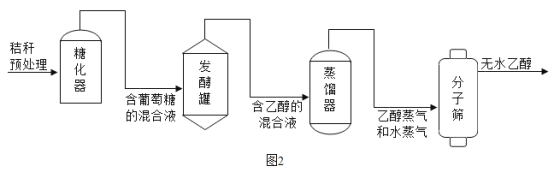
�������У�������(C6H12O6)�ھƻ�ø�Ĵ������·�����Ӧ�Ļ�ѧ����ʽΪ��C6H12O6 2C2H5OH+2X������X�Ļ�ѧʽΪ_____���÷�Ӧ����_____��Ӧ(�������Ӧ����)��
2C2H5OH+2X������X�Ļ�ѧʽΪ_____���÷�Ӧ����_____��Ӧ(�������Ӧ����)��
(4)ҽ���������75%�ľƾ���Һ�����������˾ƾ���Һ���ܼ���_____(����������)��
[��������]����100mLҽ�þƾ���������ȡ79mL 95%�ľƾ���Һ������ˮϡ����100mL��
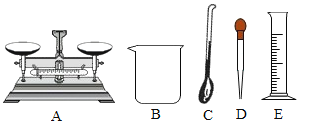
I����ȡ75mL 95%�ľƾ���Һ����ѡ�õ�������_____(��ͼ��ʾ�������)��
��С����79mL 95%�ƾ���Һ��21mL����ˮ��ϣ��������С��100mL���������Ĺ۵������ԭ��_____��
������ʦ��ָ���£�С��ѡ������ƿ(�ɾ�ȷ������һ��Ũ����Һ������)79mL 95%�ľƾ���Һ����ˮ��10mL�̶�����75%�ľƾ���Һ��
���������۶���������84����Һ����
��84����Һ��˵�����ϵIJ�����Ϣ���£�
��Ҫ�ɷ֣�NaClO
���ʳɷ֣�NaCl��NaOH
��������500mL
��Ҫ���ܣ����м��ԡ�Ư���Ժ�ʴ��
���淽�����ܷ⡢�ܹ⡢���±���
ʹ�÷�Χ��������һ������ı�������
ע���������������ϴ�Ӽ�������Һ���ʹ��
(1)NaCl��NaClO��NaOH�������ӵĽṹʾ��ͼΪ_____(�����)��
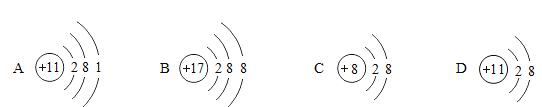
(2)�����£�������Һ��pH_____(�����)��
A ����7
B ����7
C ��7
(3)��ҵ�Ͻ�����(Cl2)ͨ���ռ���Һ�п���ȡ����Һ����Ӧ���γ���NaCl��NaClO����Һ���÷�Ӧ�Ļ�ѧ����ʽΪ_____��
(4)��84����Һ��(��Ҫ�ɷ���NaClO) �ͽ��(��Ҫ�ɷ�������) ���ʹ�û�����ж����壬���ܵ����ж���С�������ж�����������������������������_____��
(5)̽��NaClO��Ư����
[��������]
��������(NaClO) ����ˮ��Ӧ��ˮ��Һ���ͷ���������Ӧ�Ĺ���ΪNaClO+H2O=HClO+NaOH��2HClO![]() 2HCl+O2�����ù����л��ϼ۷����仯��Ԫ����_____��������(HClO) ��ʹȾ�Ϻ��л�ɫ����ɫ��������Ư����
2HCl+O2�����ù����л��ϼ۷����仯��Ԫ����_____��������(HClO) ��ʹȾ�Ϻ��л�ɫ����ɫ��������Ư����
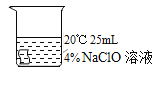
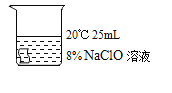
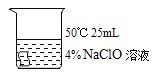
[����ʵ��]ѡ�ú�ɫ������������ͼʵ�飺
��� | �� | �� | �� | �� |
ʵ����� |
|
|
|
|
5min�� | �����Ա�dz | ������Ϊ��ɫ | ������Ϊ��ɫ | ������Ϊ���ɫ |
[���������]
I��ʵ���У����ó�������������ͬʱ�������¶ȿ�ʹ����������Һ��Ư������ǿ���Ľ��ۣ���Ҫ�Ա�_____��
ʵ��(����)��
���Է���ʵ���Ӧ���Ƶ��¶���������Ƹ�ʵ���Ŀ����_____��
����ʵ�飬���������һ��ʹ����84����Һ������ʱ��ע������_____��