��Ŀ����
ijʵ��С������10�˵�����CuSO4?5H2O����ȡ����ͭ��֤������ͭ�ܼӿ�H2O2�ķֽ⣮���������������ȡ����ͭ��ʵ�鲽�裮����ʾ��������ͭ���ȷֽ���������ͭ��ˮ��
��1����ȡ10�˵������������С�ձ��У���
��2�����裨1���е��ձ��ڵμ�һ������
��3�������裨2�����û������ˡ�ϴ�ӣ�
��4��������Һ���Ƿ�����ͭ������������
��5�����鲽�裨3����������Ƿ�ϴ�Ӹɾ�������������
��6����ϴ�Ӹɾ��Ĺ����������ֱ��ȫ����Ϊ��ɫ������ͭ��
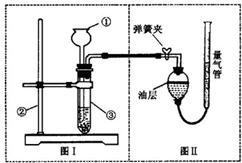
 ��С���������ͼʵ��װ����֤������ͭ�ܼӿ�˫��ˮ��������������Լ5%���ķֽⲢ��������̵Ĵ�Ч�����бȽϣ����ȽϷ�Ӧ���ʣ�����ͼʾװ�ò�������������������������Ӱ��ʵ�����ؾ��Ѻ��ԣ�����������£�
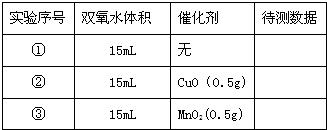
��С���������ͼʵ��װ����֤������ͭ�ܼӿ�˫��ˮ��������������Լ5%���ķֽⲢ��������̵Ĵ�Ч�����бȽϣ����ȽϷ�Ӧ���ʣ�����ͼʾװ�ò�������������������������Ӱ��ʵ�����ؾ��Ѻ��ԣ�����������£�| ��� | ˫��ˮ��� | ���� | �������� |
| �� | 15mL | �� | |
| �� | 15mL | CuO��0.5g�� | |
| �� | 15mL | MnO2��0.5g�� |
��2�������ԭ������ʵ���еġ��������ݡ�������ָ
��3��ʵ��ʱ�����ռ���ͼ�������©���У���Ҫ��֤��ȡ���������û�����ڶ���ǰӦ���еIJ�����
��4��Ϊ̽��CuO��ʵ������Ƿ�������ã�����ٱȽ��⣬���貹������ʵ�飨����д�����������a��֤��CuO�Ļ�ѧ����û�иı䣻b��
��5���������ʵ��������˫��ˮ��������������ͬ����ʵ����
��1��д��˫��ˮ������ͭ�Ĵ������·ֽ���������ķ���ʽ
��2����֪��˫��ˮ��������������Ϊ5%������������0.16g����ʵ�����ĵĹ���������Һ�����Ƕ��٣���Ҫ�м�����̣�
������I��������ͭ��ȡ����ͭʱӦ����������ͭת��Ϊ������ͭ��������ͭ�ڼ���ʱ��������ͭ����һ����Ҫ�����ܽ⡢����ϴ�ӵȣ����е�ǣ���IJ����϶ࣻ�ص���ͭ���ӡ���������ӵļ���
II����1����Ƶ�װ���г���©���¶˵�λ�ò���ȷ��
��2��������Ƶ��Dz�ͬ����ʹ��ʱ�ķ�Ӧ�ٶȱȽϣ����Ӧ�ò�����Ӧ���ٶȣ�
��3�������Եĺû��Ǽ�С��������Ҫ���أ�
��4��������������Ҫ��������ѧ��Ӧǰ��һ���������䣻���ǻ�ѧ���ʲ��䣮
��5���Ƚϵ��Dz�ͬ������Ч�����������������Ӧ����ͬ��
III������ͭ������ʱ˫��ˮ�ķֽ⣬��ʵ������ȡ�����ķ���ʽ���ƣ��ݷ���ʽ�ļ�������֪��������Ӧ�
II����1����Ƶ�װ���г���©���¶˵�λ�ò���ȷ��
��2��������Ƶ��Dz�ͬ����ʹ��ʱ�ķ�Ӧ�ٶȱȽϣ����Ӧ�ò�����Ӧ���ٶȣ�
��3�������Եĺû��Ǽ�С��������Ҫ���أ�
��4��������������Ҫ��������ѧ��Ӧǰ��һ���������䣻���ǻ�ѧ���ʲ��䣮
��5���Ƚϵ��Dz�ͬ������Ч�����������������Ӧ����ͬ��
III������ͭ������ʱ˫��ˮ�ķֽ⣬��ʵ������ȡ�����ķ���ʽ���ƣ��ݷ���ʽ�ļ�������֪��������Ӧ�
����⣺I����1������������ͭ����Һ���ʴ�Ϊ����ˮ�ܽ�
��2��������ͭת��Ϊ������ͭ�ij����������һ�ּ����Һ���ʴ�Ϊ���������Ƶ���Һ
��4��������Һ���Ƿ�������ͭ����Ҫ�ǿ���Һ���Ƿ���ͭ���ӣ��ټ�������Һ��������ɫ�������ɣ�
�ʴ�Ϊ��������Һ�еμ��������ƣ���������ɫ��������
��5�������Ƿ�ϴ�ɾ����Ǽ���ϴ��Һ���Ƿ�����������Ӵ��ڣ��ʵμ��Ȼ������Ƿ��а�ɫ�������ɼ���
�ʴ�Ϊ���ٴ�ϴ�Ӱ�ϴ�Ӻ��ˮ�еμ��Ȼ����������ް�ɫ��������
II����1����Ƶ�װ���г���©���¶˵�λ�ò���ȷ��������Һ�����������ӳ���©���ܳ���
�ʴ�Ϊ������©��Ӧ���뵽Һ������
��2����ͼ����ӳ���Ǵ�����ͬʱ����������ͬʱ��Ӧ�Ŀ��������Ӧ��ͨ��������Ӧ���ٶȣ���֤���ۣ�
�ʴ�Ϊ���ռ���ͬ��������������ʱ��
��3��Ҫ�Dz���������С�����뱣֤װ�õ������ԽϺã����������Ĵ���һ����ô���ǵ�ľ����
�ʴ�Ϊ�����װ�õ������ԣ���������ǵ�ľ�����м���
��4���ж�һ�������Ǵ��������ܸı䷴Ӧ�ٶ��⣬��Ҫ��֤��һ���������䣬�����仯ѧ���ʲ��䣮
�ʴ�Ϊ��֤������ͭ������û������
��5��ʵ���Ŀ���DZȽϲ�ͬ������Ч�����������������Ӧ����ͬ��
�ʴ�Ϊ����
III������ͭ������ʱ˫��ˮ�ķֽ⣬������ˮ���������ݷ���ʽ�ļ�������֪��������Ӧ�
�ʴ�Ϊ��2H2O2
2H2O+O2��
�裺���ĵ�˫��ˮ��Һ������X
2H2O2
2H2O+O2��
68 32
X��5% 0.16g
68��32=X��5%��0.16g
��ã�X=6.8g
����Ҫ˫��ˮ��������6.8g
��2��������ͭת��Ϊ������ͭ�ij����������һ�ּ����Һ���ʴ�Ϊ���������Ƶ���Һ
��4��������Һ���Ƿ�������ͭ����Ҫ�ǿ���Һ���Ƿ���ͭ���ӣ��ټ�������Һ��������ɫ�������ɣ�
�ʴ�Ϊ��������Һ�еμ��������ƣ���������ɫ��������
��5�������Ƿ�ϴ�ɾ����Ǽ���ϴ��Һ���Ƿ�����������Ӵ��ڣ��ʵμ��Ȼ������Ƿ��а�ɫ�������ɼ���
�ʴ�Ϊ���ٴ�ϴ�Ӱ�ϴ�Ӻ��ˮ�еμ��Ȼ����������ް�ɫ��������
II����1����Ƶ�װ���г���©���¶˵�λ�ò���ȷ��������Һ�����������ӳ���©���ܳ���
�ʴ�Ϊ������©��Ӧ���뵽Һ������
��2����ͼ����ӳ���Ǵ�����ͬʱ����������ͬʱ��Ӧ�Ŀ��������Ӧ��ͨ��������Ӧ���ٶȣ���֤���ۣ�
�ʴ�Ϊ���ռ���ͬ��������������ʱ��
��3��Ҫ�Dz���������С�����뱣֤װ�õ������ԽϺã����������Ĵ���һ����ô���ǵ�ľ����
�ʴ�Ϊ�����װ�õ������ԣ���������ǵ�ľ�����м���
��4���ж�һ�������Ǵ��������ܸı䷴Ӧ�ٶ��⣬��Ҫ��֤��һ���������䣬�����仯ѧ���ʲ��䣮
�ʴ�Ϊ��֤������ͭ������û������
��5��ʵ���Ŀ���DZȽϲ�ͬ������Ч�����������������Ӧ����ͬ��
�ʴ�Ϊ����
III������ͭ������ʱ˫��ˮ�ķֽ⣬������ˮ���������ݷ���ʽ�ļ�������֪��������Ӧ�
�ʴ�Ϊ��2H2O2
| ||
�裺���ĵ�˫��ˮ��Һ������X
2H2O2
| ||
68 32
X��5% 0.16g
68��32=X��5%��0.16g
��ã�X=6.8g
����Ҫ˫��ˮ��������6.8g
���������⿼�������ʵ�ת���������ᴿ�Լ���ͬ����Ч���ıȽϣ�ѵ����ѧ����˼ά������ǿ����ѧ�����֪ʶ��ѵ����

��ϰ��ϵ�д�
�����Ŀ
ijʵ��С������10�˵�����CuSO4?5H2O����ȡ����ͭ��֤������ͭ�ܼӿ�H2O2�ķֽ⣮
���������������ȡ����ͭ��ʵ�鲽�裮����ʾ��������ͭ���ȷֽ���������ͭ��ˮ��
��1����ȡ10�˵������������С�ձ��У���______��
��2�����裨1���е��ձ��ڵμ�һ������______��Һ�����������ij�����
��3�������裨2�����û������ˡ�ϴ�ӣ�
��4��������Һ���Ƿ�����ͭ������������______��
��5�����鲽�裨3����������Ƿ�ϴ�Ӹɾ�������������______��
��6����ϴ�Ӹɾ��Ĺ����������ֱ��ȫ����Ϊ��ɫ������ͭ��
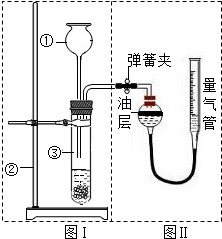
 ��С���������ͼʵ��װ����֤������ͭ�ܼӿ�˫��ˮ��������������Լ5%���ķֽⲢ��������̵Ĵ�Ч�����бȽϣ����ȽϷ�Ӧ���ʣ�����ͼʾװ�ò�������������������������Ӱ��ʵ�����ؾ��Ѻ��ԣ�����������£�
��С���������ͼʵ��װ����֤������ͭ�ܼӿ�˫��ˮ��������������Լ5%���ķֽⲢ��������̵Ĵ�Ч�����бȽϣ����ȽϷ�Ӧ���ʣ�����ͼʾװ�ò�������������������������Ӱ��ʵ�����ؾ��Ѻ��ԣ�����������£�
| ��� | ˫��ˮ��� | ���� | �������� |
| �� | 15mL | �� | |
| �� | 15mL | CuO��0.5g�� | |
| �� | 15mL | MnO2��0.5g�� |
��2�������ԭ������ʵ���еġ��������ݡ�������ָ______��
��3��ʵ��ʱ�����ռ���ͼ�������©���У���Ҫ��֤��ȡ���������û�����ڶ���ǰӦ���еIJ�����______����Ҫ���������������������Ӧ������Ƥ����______��
��4��Ϊ̽��CuO��ʵ������Ƿ�������ã�����ٱȽ��⣬���貹������ʵ�飨����д�����������a��֤��CuO�Ļ�ѧ����û�иı䣻b��______��
��5���������ʵ��������˫��ˮ��������������ͬ����ʵ����______��ѡ��С���û�С�������Ӱ�죮
��1��д��˫��ˮ������ͭ�Ĵ������·ֽ���������ķ���ʽ______��
��2����֪��˫��ˮ��������������Ϊ5%������������0.16g����ʵ�����ĵĹ���������Һ�����Ƕ��٣���Ҫ�м�����̣�
ijʵ��С������10�˵�����ȡ����ͭ��֤������ͭ�ܼӿ�H2O2�ķֽ⣮
���������������ȡ����ͭ��ʵ�鲽�裮
��1����ȡ10�˵������������С�ձ��У���______��
��2�����裨1���е��ձ��ڵμ�______��Һ��ֱ�����������ij�����
��3����������ͭ�Ƿ�������ȫ������������______
______��
��4�������裨2�����õĻ�������������ȫ����Ϊ��ɫ����ͭ��
��5���ٽ����裨4�����û������ˡ�ϴ�ӡ���ɺ���ϸ����֤�Ƿ�ϴ����������������ӵķ�����______
______��
��С���������ͼʵ��װ����֤������ͭ�ܼӿ�˫��ˮ��Ũ��Լ7%���ķֽⲢ��������̵Ĵ�Ч�����бȽϣ����ȽϷ�Ӧ���ʣ�����ͼʾװ�ò�������������������������Ӱ��ʵ�����ؾ��Ѻ��ԣ�����������£�

��1�������װ����������һ�����Ե�ȱ�ݣ�����Ϊ��ξ���______��
��2�������ԭ������ʵ���еġ��������ݡ�������ָ______��
��3��ʵ��ʱ�����ռ���ͼ�������©���У���Ҫ��֤��ȡ���������û�����ڶ���ǰӦ���еIJ�����______����Ҫ���������������������Ӧ������Ƥ����______��
��4��Ϊ̽��CuO��ʵ������Ƿ�������ã�����ٱȽ��⣬���貹������ʵ�飨����д�����������a��֤��CuO�Ļ�ѧ����û�иı䣻b��______��
��Ϊ֤������ͭ�Ļ�ѧ�����ڼ���˫��ˮǰ��û�з����ı䣬�������֤��ʵ����______��
���������������ȡ����ͭ��ʵ�鲽�裮
��1����ȡ10�˵������������С�ձ��У���______��
��2�����裨1���е��ձ��ڵμ�______��Һ��ֱ�����������ij�����
��3����������ͭ�Ƿ�������ȫ������������______
______��
��4�������裨2�����õĻ�������������ȫ����Ϊ��ɫ����ͭ��
��5���ٽ����裨4�����û������ˡ�ϴ�ӡ���ɺ���ϸ����֤�Ƿ�ϴ����������������ӵķ�����______
______��
��С���������ͼʵ��װ����֤������ͭ�ܼӿ�˫��ˮ��Ũ��Լ7%���ķֽⲢ��������̵Ĵ�Ч�����бȽϣ����ȽϷ�Ӧ���ʣ�����ͼʾװ�ò�������������������������Ӱ��ʵ�����ؾ��Ѻ��ԣ�����������£�
| ʵ����� | ˫��ˮ��� | ���� | �������� |
| �� | 15mL | �� | |
| �� | 15mL | CuO��0.5g�� | |
| �� | 15mL | MnO2��0.5g�� |

��1�������װ����������һ�����Ե�ȱ�ݣ�����Ϊ��ξ���______��
��2�������ԭ������ʵ���еġ��������ݡ�������ָ______��
��3��ʵ��ʱ�����ռ���ͼ�������©���У���Ҫ��֤��ȡ���������û�����ڶ���ǰӦ���еIJ�����______����Ҫ���������������������Ӧ������Ƥ����______��
��4��Ϊ̽��CuO��ʵ������Ƿ�������ã�����ٱȽ��⣬���貹������ʵ�飨����д�����������a��֤��CuO�Ļ�ѧ����û�иı䣻b��______��
��Ϊ֤������ͭ�Ļ�ѧ�����ڼ���˫��ˮǰ��û�з����ı䣬�������֤��ʵ����______��
��2010?������һģ��ijʵ��С������10�˵�����CuSO4?5H2O����ȡ����ͭ��֤������ͭ�ܼӿ�H2O2�ķֽ⣮
���������������ȡ����ͭ��ʵ�鲽�裮����ʾ��������ͭ���ȷֽ���������ͭ��ˮ��
��1����ȡ10�˵������������С�ձ��У���______��
��2�����裨1���е��ձ��ڵμ�һ������______��Һ�����������ij�����
��3�������裨2�����û������ˡ�ϴ�ӣ�
��4��������Һ���Ƿ�����ͭ������������______��
��5�����鲽�裨3����������Ƿ�ϴ�Ӹɾ�������������______��
��6����ϴ�Ӹɾ��Ĺ����������ֱ��ȫ����Ϊ��ɫ������ͭ��
��С���������ͼʵ��װ����֤������ͭ�ܼӿ�˫��ˮ��������������Լ5%���ķֽⲢ��������̵Ĵ�Ч�����бȽϣ����ȽϷ�Ӧ���ʣ�����ͼʾװ�ò�������������������������Ӱ��ʵ�����ؾ��Ѻ��ԣ�����������£�
��1�������װ����������һ�����Ե�ȱ�ݣ�����Ϊ��ξ���______��
��2�������ԭ������ʵ���еġ��������ݡ�������ָ______��
��3��ʵ��ʱ�����ռ���ͼ�������©���У���Ҫ��֤��ȡ���������û�����ڶ���ǰӦ���еIJ�����______����Ҫ���������������������Ӧ������Ƥ����______��
��4��Ϊ̽��CuO��ʵ������Ƿ�������ã�����ٱȽ��⣬���貹������ʵ�飨����д�����������a��֤��CuO�Ļ�ѧ����û�иı䣻b��______��
��5���������ʵ��������˫��ˮ��������������ͬ����ʵ����______��ѡ��С���û�С�������Ӱ�죮
��1��д��˫��ˮ������ͭ�Ĵ������·ֽ���������ķ���ʽ______ 2H2O+O2��

���������������ȡ����ͭ��ʵ�鲽�裮����ʾ��������ͭ���ȷֽ���������ͭ��ˮ��
��1����ȡ10�˵������������С�ձ��У���______��
��2�����裨1���е��ձ��ڵμ�һ������______��Һ�����������ij�����
��3�������裨2�����û������ˡ�ϴ�ӣ�
��4��������Һ���Ƿ�����ͭ������������______��
��5�����鲽�裨3����������Ƿ�ϴ�Ӹɾ�������������______��
��6����ϴ�Ӹɾ��Ĺ����������ֱ��ȫ����Ϊ��ɫ������ͭ��
��С���������ͼʵ��װ����֤������ͭ�ܼӿ�˫��ˮ��������������Լ5%���ķֽⲢ��������̵Ĵ�Ч�����бȽϣ����ȽϷ�Ӧ���ʣ�����ͼʾװ�ò�������������������������Ӱ��ʵ�����ؾ��Ѻ��ԣ�����������£�
| ��� | ˫��ˮ��� | ���� | �������� |
| �� | 15mL | �� | |
| �� | 15mL | CuO��0.5g�� | |
| �� | 15mL | MnO2��0.5g�� |
��2�������ԭ������ʵ���еġ��������ݡ�������ָ______��
��3��ʵ��ʱ�����ռ���ͼ�������©���У���Ҫ��֤��ȡ���������û�����ڶ���ǰӦ���еIJ�����______����Ҫ���������������������Ӧ������Ƥ����______��
��4��Ϊ̽��CuO��ʵ������Ƿ�������ã�����ٱȽ��⣬���貹������ʵ�飨����д�����������a��֤��CuO�Ļ�ѧ����û�иı䣻b��______��
��5���������ʵ��������˫��ˮ��������������ͬ����ʵ����______��ѡ��С���û�С�������Ӱ�죮
��1��д��˫��ˮ������ͭ�Ĵ������·ֽ���������ķ���ʽ______ 2H2O+O2��