��Ŀ����
���������䡱���̵Ŀ��������л������ҹ������غ���������ԴΣ�������������ǵĻ�������1����Ȼ���ڵ�һ��ͨ�������͵��û�ʱ�������Ƚ��ܵ���ע����������֪����䵪����Ŀ����______��
��2����Ȼ��������һ����ɫ��ζ�����壬����ʹ��ʱ������Ȼ���м���������������ζ��������������������______��
��3��һ����������Ȼ��й©����Ӧ��______��ȷ����ȫ��
���𰸡���������1�������ǿ�ȼ�������������ϵ�ȼ�ױ�ը��
��2����Ȼ��������ζ��й¶���ײ�����������ζ�������Է�й©��
��3��������Ȼ����������ܷ�����ը���н��
����⣺��1���κο�ȼ�������������ϵ�ȼʱ���п��ܷ�����ը�������Ļ�ѧ���ʲ����ã����ž��ܵ��ڵĿ��������������������ã�
�ʴ�Ϊ���ž��ܵ��ڵĿ�������ֹ�����������ϵ��ʱ������ը��
��2����Ȼ��������һ����ɫ����ζ�����壬й©ʱ���ڲ����
�ʴ�Ϊ���������Ǽ��緢����Ȼ��й©����ֹ��ը�¹ʵķ�����
��3��һ����������Ȼ��й©����Ӧ�ùرշ��ţ�����ͨ�磬��ȷ����ȫ���ʴ�Ϊ���رշ��ţ�����ͨ�磮
������������Ҫ��������Ȼ�������ʺ���;�ȷ����֪ʶ����������ʵ�ʵĿ����⣮
��2����Ȼ��������ζ��й¶���ײ�����������ζ�������Է�й©��
��3��������Ȼ����������ܷ�����ը���н��
����⣺��1���κο�ȼ�������������ϵ�ȼʱ���п��ܷ�����ը�������Ļ�ѧ���ʲ����ã����ž��ܵ��ڵĿ��������������������ã�
�ʴ�Ϊ���ž��ܵ��ڵĿ�������ֹ�����������ϵ��ʱ������ը��
��2����Ȼ��������һ����ɫ����ζ�����壬й©ʱ���ڲ����
�ʴ�Ϊ���������Ǽ��緢����Ȼ��й©����ֹ��ը�¹ʵķ�����
��3��һ����������Ȼ��й©����Ӧ�ùرշ��ţ�����ͨ�磬��ȷ����ȫ���ʴ�Ϊ���رշ��ţ�����ͨ�磮
������������Ҫ��������Ȼ�������ʺ���;�ȷ����֪ʶ����������ʵ�ʵĿ����⣮

��ϰ��ϵ�д�
�����Ŀ
����һ��Ҳ�벻��������������ȾӰ�������ǵ�����������ƿ������������ֿ������£�Ӫ�콡�����ʵ����滷�����ѳ�Ϊȫ����Ĺ�ʶ��
��1���������ҹ�ij������һ�ݿ�������Ԥ������
�ٴӱ�������жϣ��õ���������Ҫ������Ⱦ���� ��
�ڶ�����������Ⱦ��������Ҫ�к�����֮һ����ȥ��������ķ����ж��֣�������һ�ַ����С�ʯ��ʯ������ԭ��Ϊʯ��ʯ����ƷΪʯ�ࣨCaSO4������д����صĻ�ѧ����ʽ��
��һ�����ֽ�ʯ��ʯ ��
�ڶ�������ʯ�����������Ļ��� ��
������������������������Ӧ����ʯ�� ��
��2��һ����̼Ҳ�dz����Ĵ�����Ⱦ������������������еIJ���ȫȼ�տɱ�ʾΪ
2C8H18+23O2��12CO2+4CO+18H2O
��ij���������ȤС���ͬѧ�����������IJ���������CO�����仯���ߣ��������ʾ����ʱ��0��24Сʱ���������ʾCO������������Ϊ�ȽϷ���ʵ�ʵ�����һ��ͼ
A��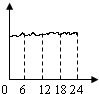 B��
B��
C�� D��
D��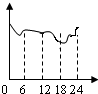
��Ŀǰ����������ȫ��ʹ�á��������䡱�������͵���Ȼ�������û��˹�ú������Ȼ���Ĺ�����һ��Ҫ��ԭ�е���߽��и�������������Ҳ���������ȫȼ�գ�����ʦ�ɼ���20.8g��Ȼ��ȼ�յIJ��ͨ����ˮ�Ȼ��ƹ��壨������������������10.8g����ԭ������һ����̼�Ͷ�����̼���������� �������費�����������壩
��3����ý�屨����Ŀǰ�Ҿӻ����Ŀ�����Ⱦ��Ҫ��װ��ʱ���������к������ļ�ȩ��CH2O�����£����ڽӴ���ȩ����Ⱥ���ڡ��ǡ��������β������ķ����ʻ��������ӣ������ϳ��۵�һ�֡���ȩ���������к��еĹ������⣨H2O2��������Ч�ؽ���ȩ����Ϊ�����壬ͬʱ����ˮ����д���ù��̵Ļ�ѧ����ʽ�� ��
��1���������ҹ�ij������һ�ݿ�������Ԥ������
| ָ �� | ����ʵ�� | ����Ԥ�� | ||
| API | ���������ȼ� | API | ���������ȼ� | |
| ������������ | 26 | �� | 20-35 | �� |
| �������� | 38 | �� | 30-45 | �� |
| �������� | 59 | �� | 55-75 | �� |
| ˵ �� | Ԥ�ƽ��տ��������Ϻã�����������Ӱ�죬������������ | |||
�ڶ�����������Ⱦ��������Ҫ�к�����֮һ����ȥ��������ķ����ж��֣�������һ�ַ����С�ʯ��ʯ������ԭ��Ϊʯ��ʯ����ƷΪʯ�ࣨCaSO4������д����صĻ�ѧ����ʽ��
��һ�����ֽ�ʯ��ʯ
�ڶ�������ʯ�����������Ļ���
������������������������Ӧ����ʯ��
��2��һ����̼Ҳ�dz����Ĵ�����Ⱦ������������������еIJ���ȫȼ�տɱ�ʾΪ
2C8H18+23O2��12CO2+4CO+18H2O
��ij���������ȤС���ͬѧ�����������IJ���������CO�����仯���ߣ��������ʾ����ʱ��0��24Сʱ���������ʾCO������������Ϊ�ȽϷ���ʵ�ʵ�����һ��ͼ
A��
 B��
B��
C��
 D��
D��
��Ŀǰ����������ȫ��ʹ�á��������䡱�������͵���Ȼ�������û��˹�ú������Ȼ���Ĺ�����һ��Ҫ��ԭ�е���߽��и�������������Ҳ���������ȫȼ�գ�����ʦ�ɼ���20.8g��Ȼ��ȼ�յIJ��ͨ����ˮ�Ȼ��ƹ��壨������������������10.8g����ԭ������һ����̼�Ͷ�����̼����������
��3����ý�屨����Ŀǰ�Ҿӻ����Ŀ�����Ⱦ��Ҫ��װ��ʱ���������к������ļ�ȩ��CH2O�����£����ڽӴ���ȩ����Ⱥ���ڡ��ǡ��������β������ķ����ʻ��������ӣ������ϳ��۵�һ�֡���ȩ���������к��еĹ������⣨H2O2��������Ч�ؽ���ȩ����Ϊ�����壬ͬʱ����ˮ����д���ù��̵Ļ�ѧ����ʽ��
 ���ǵ����������벻��ȼ�ϣ�������ѧ֪ʶ���ش��������⣺
���ǵ����������벻��ȼ�ϣ�������ѧ֪ʶ���ش��������⣺