��Ŀ����
 ���ǵ����������벻��ȼ�ϣ�������ѧ֪ʶ���ش��������⣺
���ǵ����������벻��ȼ�ϣ�������ѧ֪ʶ���ش��������⣺ͼ1��2008�걱�����˻�Ļ��ͼƬ�������ڽ���������Ѯ������ʡ�Ϸʡ����ϡ���
������Ϫ����ɽ������У����˻������ȼ�ϳ�Ϊ���飨��ѧʽΪC3H8������������
���
���
����л�������������ȼ�ղ�����������̼
������̼
���ڡ������鶨�顷���������ʣ���2��ͼ2�ǡ��������䡰-V�̵�ͼƬ�����������䡱����ʹ��ʡ���ֳ���������
��Ȼ��
��Ȼ��
����ȼ�����ƣ�������Ҫ�ɷ��ڿ�������ȫȼ�յĻ�ѧ����ʽΪCH4+2O2
CO2+2H2O
| ||
CH4+2O2
CO2+2H2O
��
| ||
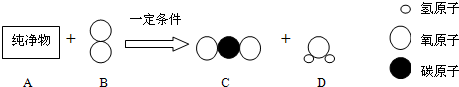
��3����̼��Ԫ����ɵĻ������У���һ�����ʽ�������������CH4Ϊ���飬C2H6Ϊ���飬
C3H8Ϊ���飬C4H10Ϊ���顭��������к���8��̼ԭ�ӵ������Ļ�ѧʽΪ
C8H18
C8H18
��������������ԭ�������࣬���к�̼Ԫ�ص���������������
����
�������С������C3H8��CH4�ֱ���ȫȼ�գ����ɵ�C02��H20�ķ�����֮�ȣ�ǰ��С��
��
���ߣ�����ڡ���С�ڡ����ڡ�������������1�����ݱ������ɺ�ȼ�յIJ��������
��2�����������䡱������Ȼ������Ҫ�ɷ��Ǽ��飬������ȫȼ�������˶�����̼��ˮ�����ݷ�Ӧд����Ӧ�ķ���ʽ��
��3��������ԭ������̼ԭ�ӵĸ����Ĺ�ϵ�����ܽἴ�ɣ�
��2�����������䡱������Ȼ������Ҫ�ɷ��Ǽ��飬������ȫȼ�������˶�����̼��ˮ�����ݷ�Ӧд����Ӧ�ķ���ʽ��
��3��������ԭ������̼ԭ�ӵĸ����Ĺ�ϵ�����ܽἴ�ɣ�
����⣺��1�����飨��ѧʽΪC3H8�����Ǻ���̼Ԫ�صĻ���������л��ȼ�յIJ����Ƕ�����̼��ˮ����ȼ�ղ����ж�����̼���ڡ������鶨�顷���������ʣ�
��2�����������䡱������Ȼ����ʹ��ʡ���ֳ�����������Ȼ��������Ҫ�ɷ��Ǽ��飬�ڿ�������ȫȼ�������˶�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH4+2O2
CO2+2H2O��
��3����̼ԭ�Ӹ�������ԭ�Ӹ����Ĺ�ϵ���ɷ���ÿ����1��̼ԭ�ӣ���ԭ�Ӹ�������2������ô��8��̼ԭ�ӵ����к�����ԭ�Ӹ���Ӧ�ñȼ�����̼ԭ�Ӷ�7������ԭ�Ӷ�14��������ѧʽΪC8H18��������������ԭ�������࣬���к�̼Ԫ�ص���������������C3H8��CH4�ֱ���ȫȼ�գ����ɵ�C02��H20�ķ�����֮�ȣ�ǰ��С�ں��ߣ�
�ʴ�Ϊ����1���л��������̼����2����Ȼ����CH4+2O2
CO2+2H2O����3��C8H18�������٣�
��2�����������䡱������Ȼ����ʹ��ʡ���ֳ�����������Ȼ��������Ҫ�ɷ��Ǽ��飬�ڿ�������ȫȼ�������˶�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH4+2O2
| ||
��3����̼ԭ�Ӹ�������ԭ�Ӹ����Ĺ�ϵ���ɷ���ÿ����1��̼ԭ�ӣ���ԭ�Ӹ�������2������ô��8��̼ԭ�ӵ����к�����ԭ�Ӹ���Ӧ�ñȼ�����̼ԭ�Ӷ�7������ԭ�Ӷ�14��������ѧʽΪC8H18��������������ԭ�������࣬���к�̼Ԫ�ص���������������C3H8��CH4�ֱ���ȫȼ�գ����ɵ�C02��H20�ķ�����֮�ȣ�ǰ��С�ں��ߣ�
�ʴ�Ϊ����1���л��������̼����2����Ȼ����CH4+2O2
| ||
������������Ҫ���黯ѧʽ�����塢���ݻ�ѧʽȷ���������Ԫ��֮�����������ϵ��ѧ�������е���Ϣ�ҹ��ɵķ���

��ϰ��ϵ�д�
 ȫ��������ϵ�д�
ȫ��������ϵ�д� һ��һ����ʱ���ϵ�д�
һ��һ����ʱ���ϵ�д�
�����Ŀ

 ���ǵ����������벻��ȼ�ϣ�������ѧ֪ʶ���ش��������⣺
���ǵ����������벻��ȼ�ϣ�������ѧ֪ʶ���ش��������⣺