��Ŀ����
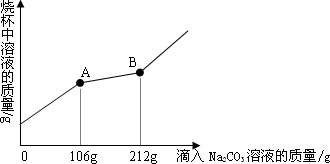
��һ�ձ���ʢ��100 gCuSO4��H2SO4�Ļ����Һ�������μ�������������Ϊ10%��NaOH��Һ�������Һ��������������NaOH��Һ��������ϵ��������ͼ��ʾ�����������ش���������![]() ��
��
��1����ʵ������в�����������������______ g��
��2����ʵ������м���80 gNaOH��Һʱ������Һ��pH��___ _7 (����ڡ���С�ڡ�����
��3����ʵ������м���80 gNaOH��Һʱ��ͨ���������ʱ���ò�������Һ�����ʵ���������������������ȷ��0.1%��

����������ͼ��֪��NaOH��Һ��������0~40 g֮�䲢û�г������������ȷ����ķ�Ӧ�ǣ�2NaOH+H2SO4=Na2SO4+2H2O��NaOH��Һ��������40 g~80 g֮�䣬�����������ż���Na![]() OH��Һ���������Ӷ��ﵽ���ֵ���ʷ�����2NaOH+CuSO4=Na2SO4+Cu(OH)2������NaOH��Һ����80 gʱ��������Ӧ�պý�����������Һ������ֻ��������������Ϊ����������Ӧ����������������֮�͡�
OH��Һ���������Ӷ��ﵽ���ֵ���ʷ�����2NaOH+CuSO4=Na2SO4+Cu(OH)2������NaOH��Һ����80 gʱ��������Ӧ�պý�����������Һ������ֻ��������������Ϊ����������Ӧ����������������֮�͡�
�𰸣���1��4.9 g����2�����ڣ�
��3���⣺�������ᷴӦ���ɵ�̼��������Ϊx��������ͭ��Ӧ���ɵ�����������Ϊy��
2NaOH+H2SO4=Na2SO4+2H2O������������������������
�������� ��80��������142
������40 g��10%���� x
������������������������������������
��ã�x=7.1 g��
2NaOH + CuSO4 = Na2SO4+�� Cu(OH)2������������
���������� 80������������������ 142
���� ��80 g��40 g����10%�������� y
��ã�y=7.1 g��
��������Һ��Na2SO4������Ϊ�� 7.1 g+7.1 g = 14.2 g��
��������Һ������Ϊ�� 100 g+80 g��4.9 g = 175.1 g��
���ò�������Һ��Na2SO4����������![]() ��100%=8.1%��
��100%=8.1%��
���ԡ�
���״���㾦�������ǰ���������⸴�ӣ�����Ĺؼ��Ǹ���ͼ��ķֶΣ��ܹ���ʶ����Ӧ�������Ⱥ�˳���ܹ����⼸�������Ļ�ѧ���塣��������Һ������������ʱ�������Ը���Ԫ���غ�˼�룬����H2SO4��Na2SO4�Ķ�Ӧ��ϵ�����������������һ�����������������

 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�