��Ŀ����
ijѧϰС���ͬѧ��ѧϰ��Na2CO3��NaHCO3�����ʺ��˽���Ƕ��������ᷴӦ����CO2���壬��ô��μ���Na2CO3��NaHCO3�أ��������������ʣ����ǽ���������̽����[��������]
��1��Ca��HCO3��2������ˮ��
��2��NaHCO3�������ȷֽ�����̼���ơ�������̼��ˮ��
[����]
��1����ͬѧ��Ϊ���ó���ʯ��ˮ����Na2CO3��NaHCO3��Һ��
��2����ͬѧ��Ϊ����CaCl2��Һ����Na2CO3��NaHCO3��Һ��
�ס�����ͬѧ�IJ��������ݳ���ʯ��ˮ��CaCl2��Һ�ֱ���Na2CO3��Һ��Ӧ���г�����������֪��Ca��HCO3��2������ˮ����˲²����ʯ��ˮ��CaCl2��Һ�ֱ���NaHCO3��Һ��ϲ�������������Ӷ������������Һ��
��3����ͬѧ��Ϊ����Na2CO3��NaHCO3���ü��ȵķ�������
[ʵ��̽��]
��1����ͬѧ����֧�ֱ�ʢ������Na2CO3��NaHCO3��Һ���Թ��У����������ʯ��ˮ���۲쵽��֧�Թ��е�������ͬ���������˰�ɫ������ʵ��������벻һ�£��������ó���ʯ��ˮ����Na2CO3��NaHCO3��Һ��
��2����ͬѧ��CaCl2��Һ���뵽�ֱ�ʢ������Na2CO3��NaHCO3��Һ���Թ��У�������֧�Թ���Ҳ�������˰�ɫ������ʵ�����������ϣ������ݹ۲쵽������ʵ����������Ϊ�Կ���CaCl2��Һ����Na2CO3��NaHCO3��Һ��
��3����ͬѧ�ֱ�ȡ��һ������Na2CO3��NaHCO3�����ڴ��Թ��м��ȣ���ͼ1����
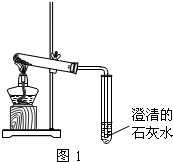
�ټ���Na2CO3ʱ����ʼ����С�Թ������������ݲ������������ȣ��������٣�δ������ʯ��ˮ����ǣ�
�ڼ���NaHCO3ʱ����ͬѧ�۲쵽ʵ��������ٲ�ͬ��֤ʵ���Լ��IJ����Ǻ����ģ�
[��������]
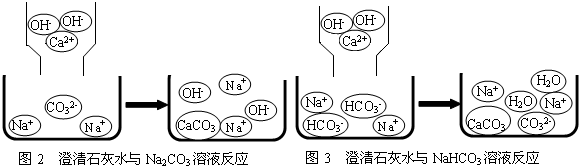
��1��С��ͬѧ������ʵ��չ�������ۣ��Լ�ͬѧ������ʵ������˱Ƚϣ�������������Һ�н������ͬ���ӵ��۽Ƕȷ�����ԭ����ͼ2��ͼ3������д��Na2CO3�����ʯ��ˮ������Ӧ�Ļ�ѧ����ʽ
��2����ͬѧ������ʵ���У���Ҷ�CaCl2��Na2CO3��Һ�ķ�Ӧ�Ƚ���Ϥ���÷�Ӧ�Ļ�ѧ����ʽΪ
����CaCl2��NaHCO3��ҺΪʲô�ܷ���������Ӧ����ʦָ���䷴Ӧԭ���ϸ��ӣ��д��ڽ��ѧϰ�н�һ��̽����
��3����ͬѧ�ڼ���Na2CO3����ʱ����ʼ�����������ݵ�ԭ����
[��չӦ��]
��1������Na2CO3�л�������NaHCO3����ͨ��
��2��Ҫ��ȥNa2CO3��Һ�л��е�����NaHCO3���ɼ���������
������[��������]��1��ͨ������ͼ���йط�Ӧ����ǰ�����ӵı仯������д����Ӧ����ʽ�Ͳ��뷴Ӧ�����ӣ�
��2������������ϢCaCl2+2NaHCO3=CaCO3��+2NaCl+CO2��+H2O����֪�÷�Ӧ���а�ɫ�������������������̼���ƺ�
CaCl2��Һ��Ӧֻ�г������ɣ�
[��չӦ��]��1����Ϊ̼�����Ʋ��ȶ������ü��Ƚ��г��ӣ�
��2�����Բ���ת������̼������ת��Ϊ̼���ƣ�
��2������������ϢCaCl2+2NaHCO3=CaCO3��+2NaCl+CO2��+H2O����֪�÷�Ӧ���а�ɫ�������������������̼���ƺ�
CaCl2��Һ��Ӧֻ�г������ɣ�
[��չӦ��]��1����Ϊ̼�����Ʋ��ȶ������ü��Ƚ��г��ӣ�
��2�����Բ���ת������̼������ת��Ϊ̼���ƣ�
����⣺[��������]
��1����ͼ2��֪Na2CO3��Ca��OH��2��Ϻ���������CaCO3���ɼ����������Ӻ������������Է���ʽΪ��Na2CO3+Ca��OH��2=CaCO3��+2NaOH��
��ͼ3��֪NaHCO3�����ʯ��ˮ��ϣ���Ӧǰ��������HCO3-Na+Ca2+ OH-����Ӧ����������CaCO3��H2O��Na+��CO32-���Ա�ǰ���֪�μӷ�Ӧ��������OH-��HCO3-��Ca2+
��2��CaCl2��Na2CO3��Һ�ķ������ֽⷴӦ������ʽΪNa2CO3+CaCl2=CaCO3��+2NaCl
CaCl2��NaHCO3��Һ��ϣ�����������ϢCaCl2+2NaHCO3=CaCO3��+2NaCl+CO2��+H2O����֪�÷�Ӧ���а�ɫ�������������������̼���ƺ�CaCl2��Һ��Ӧֻ�г������ɣ�������ͬѧ�Ǹ����������ݲ������������ģ�
��3��̼�����������ֽ⣬2NaHCO3
Na2CO3+CO2��+H2O�����ɵ�CO2����ͨ�����ʯ��ˮ�з�����Ӧ��
CO2+Ca��OH��2=CaCO3��+H2O ����̼��Ƴ�������ǣ�
[��չӦ��]
��1����������NaHCO3�IJ��ȶ��ԣ����ü��ȷ�������ת��Ϊ̼���ƣ�2NaHCO3
Na2CO3+CO2��+H2O
��2������������NaOH��Һ������ת��ΪNa2CO3������ʽΪ NaHCO3+NaOH=Na2CO3+H2O
�ʴ�Ϊ��
[��������]
��1��Na2CO3+Ca��OH��2=CaCO3��+2NaOH�� OH-��HCO3-��Ca2+
��2��Na2CO3+CaCl2=CaCO3��+2NaCl���������ݲ�����
��3�������������ͣ����ף�����ʯ��ˮ����ǣ���С�Թ����д������ݲ�������Թܹܿ���ˮ��ȣ���
[��չӦ��]
��1������
��2��NaOH
��1����ͼ2��֪Na2CO3��Ca��OH��2��Ϻ���������CaCO3���ɼ����������Ӻ������������Է���ʽΪ��Na2CO3+Ca��OH��2=CaCO3��+2NaOH��
��ͼ3��֪NaHCO3�����ʯ��ˮ��ϣ���Ӧǰ��������HCO3-Na+Ca2+ OH-����Ӧ����������CaCO3��H2O��Na+��CO32-���Ա�ǰ���֪�μӷ�Ӧ��������OH-��HCO3-��Ca2+
��2��CaCl2��Na2CO3��Һ�ķ������ֽⷴӦ������ʽΪNa2CO3+CaCl2=CaCO3��+2NaCl
CaCl2��NaHCO3��Һ��ϣ�����������ϢCaCl2+2NaHCO3=CaCO3��+2NaCl+CO2��+H2O����֪�÷�Ӧ���а�ɫ�������������������̼���ƺ�CaCl2��Һ��Ӧֻ�г������ɣ�������ͬѧ�Ǹ����������ݲ������������ģ�
��3��̼�����������ֽ⣬2NaHCO3
| ||
CO2+Ca��OH��2=CaCO3��+H2O ����̼��Ƴ�������ǣ�
[��չӦ��]
��1����������NaHCO3�IJ��ȶ��ԣ����ü��ȷ�������ת��Ϊ̼���ƣ�2NaHCO3
| ||
��2������������NaOH��Һ������ת��ΪNa2CO3������ʽΪ NaHCO3+NaOH=Na2CO3+H2O
�ʴ�Ϊ��
[��������]
��1��Na2CO3+Ca��OH��2=CaCO3��+2NaOH�� OH-��HCO3-��Ca2+
��2��Na2CO3+CaCl2=CaCO3��+2NaCl���������ݲ�����
��3�������������ͣ����ף�����ʯ��ˮ����ǣ���С�Թ����д������ݲ�������Թܹܿ���ˮ��ȣ���
[��չӦ��]
��1������
��2��NaOH
������������Ϣ�����Ķ����ܴ����ȱ�����������Ϣ���ٽ�Ͽα�����֪ʶ���ٽ��⣮

��ϰ��ϵ�д�
 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
�����Ŀ
 ʵ���ǻ�ѧ����꣬��ѧ�û�ѧ����Ҫ���ڣ�
ʵ���ǻ�ѧ����꣬��ѧ�û�ѧ����Ҫ���ڣ�