��Ŀ����
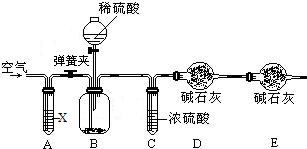
��֪ij���������к��������Ȼ��ƣ�Ϊ�ⶨ�����д��������������������ͼװ�ý���ʵ�飮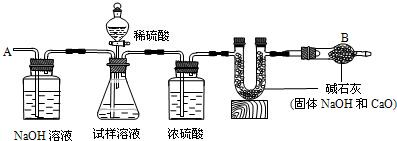
��Ҫ�������£�����գ�
�ٰ�ͼ��װ�����������
�ڽ�10g����������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ��
�۳���ʢ�м�ʯ�ҵ�U�ιܵ�����Ϊ300g��
�ܴӷ�Һ©���е���20%��ϡ���ᣬֱ��
�ݴӵ���A����������һ�����Ŀ�����
���ٴγ���ʢ�м�ʯ�ҵ�U�ιܵ�������
���ظ��ݺ͢IJ�����ֱ��U�ιܵ������������䣬�������Ϊ303.3g��
�ش��������⣺
��1��װ����Ũ�����������
��2������ݵ�Ŀ����
��3�������д������������Ϊ
��4����Һ©���е�ϡH2SO4���ܻ���Ũ���ᣬ������
��5���������ɳ����ķ������ⶨ�����д��������������Ӧѡ�õ��Լ���
�������ٴ�ʵ�鲽����з������ܴ�̼�������ᷴӦ��ʵ��������з��������ݸ��ֽⷴӦ�Ĺ���д����Ӧ�Ļ�ѧ����ʽ��
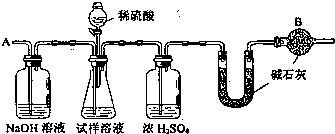
��1����Ũ�������;������
��2����Ӧ���װ���ڻ��в���Ķ�����̼���ܽ���U�ܣ�
��3������������������=
��100%��������������֪������Ҫ��������������U��ǰ�����ӵ������Ƿ�Ӧ���ɶ�����̼�����������ݶ�����̼����������������������
��4����Ũ��������ʷ�����
��5����̼������ӵ��������ӶԽ��з�����
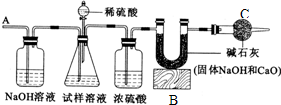
��1����Ũ�������;������
��2����Ӧ���װ���ڻ��в���Ķ�����̼���ܽ���U�ܣ�
��3������������������=
| ��������� |
| ���������� |
��4����Ũ��������ʷ�����
��5����̼������ӵ��������ӶԽ��з�����
����⣺��װ�����Ӻú�Ӧ�ȼ��װ�õ������ԣ���װ��©����������������ݻ��������װ�ã��ʴ�Ϊ��װ�õ�������
��̼���������ᷴӦ���������ɣ������ٲ�������ʱ˵��̼�����ѷ�Ӧ�ꣻ̼���������ᷴӦ���������ơ�ˮ�Ͷ�����̼��
�ʴ�Ϊ�����ٲ������壻 Na2CO3+H2SO4=Na2SO4+CO2��+H2O
��1��Ũ���������ˮ�ԣ����������������Ũ����������dz�ȥ������̼�е�ˮ��������ֹˮ��������U���У��ʴ�Ϊ����ȥˮ
��2��Ϊ��ʹװ���ڲ���Ķ�����̼Ҳȫ������U�ܣ�Ӧ�ӵ���A����������һ�����Ŀ������ʴ�Ϊ��ʹ��Ӧ�����Ķ�����̼ȫ������U�ι�
��3���贿�������Ϊx
Na2CO3+H2SO4=Na2SO4+CO2��+H2O
106 44
x 303.3g-300g=3.3g
=
��x=7.95g
��100%=79.5%
�ʴ�Ϊ��79.5%
��4��Ũ������лӷ��ԣ��ӷ������Ȼ�������һ���ֻᱻU���еļ�ʯ�����գ��Բⶨ�����Ӱ�죮�ʴ�Ϊ��Ũ�����лӷ��ԣ��Բⶨ�����Ӱ�죮
��5����̼���Ʒ�Ӧ���ɳ���������ȫ������ˮ�����ɵij���ֻ����̼���γ�����̼����������������ӻ����Ӷ������ɳ��������Կ����Եĺ������ӻ����ӵ��λ��У�
�ʴ�Ϊ���Ȼ��ƻ��Ȼ���������ƻ����ᱵ���������ƻ����������ȵȣ��������⼴�ɣ�
��̼���������ᷴӦ���������ɣ������ٲ�������ʱ˵��̼�����ѷ�Ӧ�ꣻ̼���������ᷴӦ���������ơ�ˮ�Ͷ�����̼��
�ʴ�Ϊ�����ٲ������壻 Na2CO3+H2SO4=Na2SO4+CO2��+H2O
��1��Ũ���������ˮ�ԣ����������������Ũ����������dz�ȥ������̼�е�ˮ��������ֹˮ��������U���У��ʴ�Ϊ����ȥˮ
��2��Ϊ��ʹװ���ڲ���Ķ�����̼Ҳȫ������U�ܣ�Ӧ�ӵ���A����������һ�����Ŀ������ʴ�Ϊ��ʹ��Ӧ�����Ķ�����̼ȫ������U�ι�
��3���贿�������Ϊx
Na2CO3+H2SO4=Na2SO4+CO2��+H2O
106 44
x 303.3g-300g=3.3g
| 106 |
| 44 |
| x |
| 3.3g |
| 7.95g |
| 10g |
�ʴ�Ϊ��79.5%
��4��Ũ������лӷ��ԣ��ӷ������Ȼ�������һ���ֻᱻU���еļ�ʯ�����գ��Բⶨ�����Ӱ�죮�ʴ�Ϊ��Ũ�����лӷ��ԣ��Բⶨ�����Ӱ�죮
��5����̼���Ʒ�Ӧ���ɳ���������ȫ������ˮ�����ɵij���ֻ����̼���γ�����̼����������������ӻ����Ӷ������ɳ��������Կ����Եĺ������ӻ����ӵ��λ��У�
�ʴ�Ϊ���Ȼ��ƻ��Ȼ���������ƻ����ᱵ���������ƻ����������ȵȣ��������⼴�ɣ�
��������ʵ���װ������˳��dz����ܣ���ǰ�������������Һ��Ϊ�˷�ֹ�����еĶ�����̼����װ�ã������ļ�ʯ��Ҳ��Ϊ�˷�ֹ�����еĶ�����̼����װ�ã�

��ϰ��ϵ�д�
 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
�����Ŀ