��Ŀ����
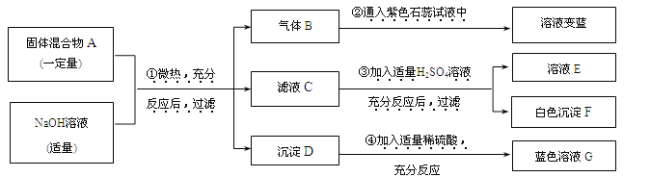
����Ŀ������һƿ���õı�����������Ϊ10����NaOH��Һ��Ʒ��Ϊ̽������ʣ�NaOH�Ϳ����е�CO2��Ӧ����Na2CO3����������⣬����U�ι��������ͼ��ʾ��װ�ý���ʵ�顣
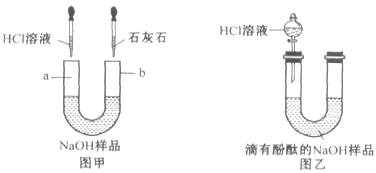
��1����ͼ����ʾ��������Һ���ʣ��ֱ���U�ιܵ����˵���HCl��Һ�ͳ����ʯ��ˮʱ���۲쵽������a��_____________________��b��___________________��
��2����ͼ����ʾ��ȡ��NaOH��Ʒ��Һ20g����U�ι��У��������м��������ķ�̪��Һ��ͨ����Һ©����U�ι��е���HCl��Һ��������20gHCl��Һʱ��ǡ�÷�Ӧ��ȫ��
�ٵ���ϡ��������У�U�ι��е���Һ��ɫ�仯Ϊ______________________________��
��ǡ����ȫ��Ӧʱ����������0��22g���Լ������Һ��Na2CO3������������
���𰸡���1������ɫ�������� �а�ɫ�������ɣ�2���� ��Һ�ɺ�ɫ��Ϊ��ɫ ��2.65%
��������
�����������ͼ����ʾ��������Һ���ʣ��ֱ���U�ιܵ����˵���HCl��Һ�ͳ����ʯ��ˮʱ���۲쵽����������ɫ�������ɣ�b���а�ɫ�������ɣ���Ϊ�������Ʊ��ʺ��Ϊ̼���ƻ������ ��Ӧ����������̼���壬ͬʱ�����ʯ��ˮ��Ӧ��������̼��ƣ��ٵ���ϡ��������У�U�ι��е���Һ��ɫ�仯Ϊ��ʼ������������ƹ�����������Һ����ɫΪ��ɫ����������IJ��ϼ��룬��������Խ��Խ�٣���Һ��ɫ������dz������Ӧǡ�û��������������Һ�ı�Ϊ��ɫ��
�⣺�����Һ��Na2CO3������ΪX
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
X 0��22g
106��44=X��0��22g
X=0��53g
�����Һ��Na2CO3����������Ϊ��0.53�ˣ�20�ˡ�100%= 2��65��