��Ŀ����
���ͼ�е�ʵ��װ�ûش�������⣺
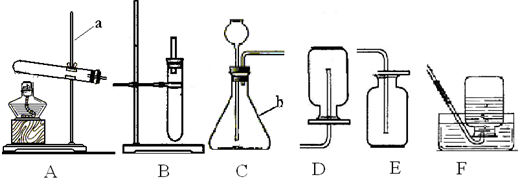
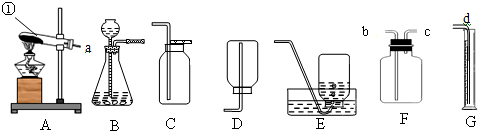
��1��д��ͼ��a��b���������ƣ�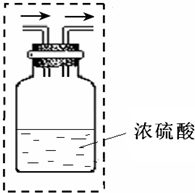
��2����˫��ˮ��O2�Ļ�ѧ����ʽΪ
��3��ʵ������������Ʊ�����Ӧ��ѡ��ķ���װ����
��4��ʵ���ҳ������������ƣ�Na2SO3��������Ũ���ᷴӦ����ȡSO2��
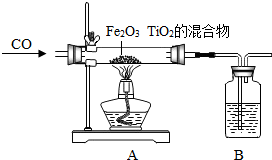
����֪��SO2��һ��û����ɫ���д̼�����ζ���ж����壬������ˮ���ܶȱȿ��������������ڿ����в���Ӧ����ô���ռ�SO2��װ��Ϊͼ�е�
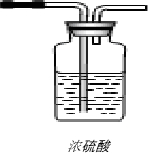
��Ϊ�˵õ�����Ķ����������壬������ˮ�����Ķ�����������ͨ��Ũ���ᣨ��ͼ���������װ��Ũ�����Լ�ƿ�ڵĵ��ܲ���������ͼ�С�������ʾ��������

��1��д��ͼ��a��b���������ƣ�
����̨
����̨
����ƿ
��ƿ
��
��2����˫��ˮ��O2�Ļ�ѧ����ʽΪ
2H2O2
2H2O+O2����
| ||
2H2O2
2H2O+O2����
�����μӷ�Ӧ��H2O2��������34g�����ɵ�������������
| ||
16
16
g����3��ʵ������������Ʊ�����Ӧ��ѡ��ķ���װ����
A
A
��������ĸ����4��ʵ���ҳ������������ƣ�Na2SO3��������Ũ���ᷴӦ����ȡSO2��
����֪��SO2��һ��û����ɫ���д̼�����ζ���ж����壬������ˮ���ܶȱȿ��������������ڿ����в���Ӧ����ô���ռ�SO2��װ��Ϊͼ�е�
E
E
������ĸ������Ϊ�˵õ�����Ķ����������壬������ˮ�����Ķ�����������ͨ��Ũ���ᣨ��ͼ���������װ��Ũ�����Լ�ƿ�ڵĵ��ܲ���������ͼ�С�������ʾ��������
��������1����Ϥ�����������˽����ƣ�
��2������˫��ˮ��ȡ�����ķ�Ӧԭ����д����ʽ�������м��㣻
��3������ʵ������������Ʊ������ǹ��������ȡ����ѡ����װ�ã�
��4�����ݶ��������ܶȺ��ܽ���ѡ���ռ�װ�ã�������ˮ�����Ķ�����������ͨ��Ũ���ᣬ��Ҫ�����ܵ��ϳ������ҵ��ĵ�����Ҫ����Һ���У�
��2������˫��ˮ��ȡ�����ķ�Ӧԭ����д����ʽ�������м��㣻
��3������ʵ������������Ʊ������ǹ��������ȡ����ѡ����װ�ã�
��4�����ݶ��������ܶȺ��ܽ���ѡ���ռ�װ�ã�������ˮ�����Ķ�����������ͨ��Ũ���ᣬ��Ҫ�����ܵ��ϳ������ҵ��ĵ�����Ҫ����Һ���У�
����⣺��1��ͼ��a������̨��b����ƿ��
�ʴ�Ϊ������̨����ƿ��
��2�����������ڶ�������������������������ˮ������������ʽ��2H2O2
2H2O+O2����
�����ɵ������������� X
2H2O2
2H2O+O2��
68 32
34g X
=
X=16g
�ʴ�Ϊ��2H2O2
2H2O+O2����16g��
��3�����������ȡ����ʱ����Ҫ���ȣ����ڡ���������͡�������ѡ��Aװ�ã�
�ʴ�Ϊ��A��
��4���ٶ�������������ˮ���ܶȱȿ����ʲ�������ˮ���ռ���ֻ���������ſ������ռ���
��ѡE��
��Ҫ�õ�����Ķ����������壬������ˮ�����Ķ�����������ͨ��Ũ����ʱ����Ҫ�����ܵ��ϳ������ҵ��ĵ�����Ҫ����Һ���У���ͼʾ��
�ʴ�Ϊ������̨����ƿ��
��2�����������ڶ�������������������������ˮ������������ʽ��2H2O2
| ||
�����ɵ������������� X
2H2O2
| ||
68 32
34g X
| 68 |
| 34g |
| 32 |
| X |
X=16g
�ʴ�Ϊ��2H2O2
| ||
��3�����������ȡ����ʱ����Ҫ���ȣ����ڡ���������͡�������ѡ��Aװ�ã�
�ʴ�Ϊ��A��
��4���ٶ�������������ˮ���ܶȱȿ����ʲ�������ˮ���ռ���ֻ���������ſ������ռ���
��ѡE��
��Ҫ�õ�����Ķ����������壬������ˮ�����Ķ�����������ͨ��Ũ����ʱ����Ҫ�����ܵ��ϳ������ҵ��ĵ�����Ҫ����Һ���У���ͼʾ��

�����������ܽϺõĿ���ѧ��Ӧ��֪ʶ����������������ȷװ��ѡȡ��������Ӧԭ���������֪ʶ����˳�����

��ϰ��ϵ�д�
 ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�
�����Ŀ
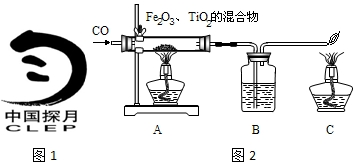
 ���϶�һ�š��ijɹ����䣬ʹȫ�����������裮��ͬѧ���Ķ����к��켼���еĻ�ѧ���IJ��ش��й����⣮
���϶�һ�š��ijɹ����䣬ʹȫ�����������裮��ͬѧ���Ķ����к��켼���еĻ�ѧ���IJ��ش��й����⣮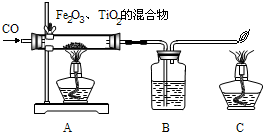
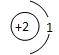 D��ԭ�ӽṹʾ��ͼΪ
D��ԭ�ӽṹʾ��ͼΪ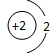
 ���϶�һ�š����ҹ��������ơ��ɹ�����ĵ�һ������̽���������϶�һ�š��ijɹ����䣬ʹȫ�����������裮��ͬѧ���Ķ����ж��IJ��ش��й����⣮
���϶�һ�š����ҹ��������ơ��ɹ�����ĵ�һ������̽���������϶�һ�š��ijɹ����䣬ʹȫ�����������裮��ͬѧ���Ķ����ж��IJ��ش��й����⣮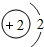 D�����ڽ���Ԫ�أ��ڻ�ѧ��Ӧ���õ�����
D�����ڽ���Ԫ�أ��ڻ�ѧ��Ӧ���õ�����