��Ŀ����
 �ڻ��������У�ԭ����������ȫ��ת���ɲ�Ʒ��ijУѧ���������Ĺ�ҵ�����ռ�����ʳɷֽ����о������Dz������ϵ�֪���ڹ�ҵ����ʳ�κ�ˮ��ɱ���ʳ��ˮ��ͨ����⣨ͨ�磩�Ƶ��ռ���Һ���ٽ���ҺŨ�����ɵõ������ռͨ������л��ɵõ�������������Cl2���������Ʋ�ù�ҵ�����ռ��п��ܺ���̼���ơ��Ȼ��ƣ��������ʵ������֤���裮
�ڻ��������У�ԭ����������ȫ��ת���ɲ�Ʒ��ijУѧ���������Ĺ�ҵ�����ռ�����ʳɷֽ����о������Dz������ϵ�֪���ڹ�ҵ����ʳ�κ�ˮ��ɱ���ʳ��ˮ��ͨ����⣨ͨ�磩�Ƶ��ռ���Һ���ٽ���ҺŨ�����ɵõ������ռͨ������л��ɵõ�������������Cl2���������Ʋ�ù�ҵ�����ռ��п��ܺ���̼���ơ��Ȼ��ƣ��������ʵ������֤���裮��1����������������ʵ�鱨�棺
| ʵ�鲽�� | ʵ������ | �� �� |
| ��ȡ������������ˮ | ������ȫ�ܽ� | -- |
| �����Թ�ȡ������Һ���������ϡ���ᣬ���ϴ����ܵĵ������������ܵ���һ�˲��� |
�� �� |
֤��ԭ�����ռ��к���̼���� |
| �����Թ����ټ��������� | �а�ɫ�������� | ֤��ԭ�����ռ��к��� |
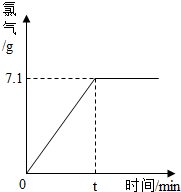
�� 3 ����ȡ100g�����µı���ʳ��ˮ����ʱ��������ԼΪ26.5%�����е�⣬����Ӧֹͣ������������������ʱ��Ĺ�ϵ��ͼ��ʾ������㣺�μӷ�Ӧ���Ȼ��Ƶ������Ƕ��٣���Ӧ�������ռ���Һ�����������Ƕ��٣�����������ȷ��0.001��
��������1�����ݼ���̼����ʱ����ͳ���ʯ��ˮ�������Ȼ�������������������ͽ��ۣ�ע��ʵ����Ҫ�ų��������ӵĸ��ţ�
��2�����ݷ�Ӧ��������ﰴ�������غ㶨����д��ѧ����ʽ��
��3�����ݻ�ѧ����ʽ���м��㣬����ͼ���֪�������������������Ը�������������������μӷ�Ӧ���Ȼ��Ƶ����������ɵ��������������������������������������Һ����������������
��2�����ݷ�Ӧ��������ﰴ�������غ㶨����д��ѧ����ʽ��
��3�����ݻ�ѧ����ʽ���м��㣬����ͼ���֪�������������������Ը�������������������μӷ�Ӧ���Ȼ��Ƶ����������ɵ��������������������������������������Һ����������������
����𣺣�1�������ռ��п��ܺ���̼���Ƶķ������ü�������Һ�������ɵ������ܷ�ʹʯ��ˮ����ǣ�Ϊ�˲�Ӱ���������Ȼ��ƣ���ѡ���������̼���ƣ��������ɶ�����̼���жϺ���̼���ƣ������Ȼ����������������������Ȼ��Ʒ�Ӧ���ɰ�ɫ���Ȼ���������
�ʴ�Ϊ��
��2����������ṩ����Ϣ�������غ㶨�ɣ���ҵ���õ�ⱥ��ʳ��ˮ���ռ�Ļ�ѧ����ʽΪ��2NaCl+2H2O
2NaOH+H2��+Cl2����
�ʴ�Ϊ��2NaCl+2H2O
2NaOH+H2��+Cl2��
��3����������������������ʱ��Ĺ�ϵ��ͼ������������������������Ϊ7.1g��
��μӷ�Ӧ��NaCl������Ϊx������H2������Ϊy�������������Ƶ�����Ϊz
2NaCl+2H2O
Cl2��+H2��+2NaOH
117 71 2 80
x 7.1g y z
=
x=11.7g
=
y=0.2g
=
z=8.0g
��Ӧ��NaOH��Һ��NaOH����������Ϊ��
��100%=8.63%
�𣺲μӷ�Ӧ���Ȼ�����11.7�ˣ���Ӧ��������Һ�����ʵ�����������8.63%��
�ʴ�Ϊ��
| ʵ�鲽�� | ʵ������ | �� �� |
| �ڳ���ʯ��ˮ | ���� ��ɫ���� |
|
| �� | ֤ �Ȼ��� |
| ||
�ʴ�Ϊ��2NaCl+2H2O
| ||
��3����������������������ʱ��Ĺ�ϵ��ͼ������������������������Ϊ7.1g��
��μӷ�Ӧ��NaCl������Ϊx������H2������Ϊy�������������Ƶ�����Ϊz
2NaCl+2H2O
| ||
117 71 2 80
x 7.1g y z
| 117 |
| 71 |
| x |
| 7.1g |
| 71 |
| 2 |
| 7.1g |
| y |
| 71 |
| 80 |
| 7.1g |
| z |
��Ӧ��NaOH��Һ��NaOH����������Ϊ��
| 8.0g |
| 100g-7.1g-0.2g |
�𣺲μӷ�Ӧ���Ȼ�����11.7�ˣ���Ӧ��������Һ�����ʵ�����������8.63%��
������������Ҫ���麬�������ʼ��鷽���ͻ�ѧ����ʽ�ļ��㼰�������������ļ��㣬�ѶȽϴ�
ע�⣺���㷴Ӧ����Һ����ʱ���÷�Ӧǰ��Һ������ȥ�������������ʱ������Ҫ��ȥ��������������Ҫ��ȥ������������
ע�⣺���㷴Ӧ����Һ����ʱ���÷�Ӧǰ��Һ������ȥ�������������ʱ������Ҫ��ȥ��������������Ҫ��ȥ������������

��ϰ��ϵ�д�
 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
�����Ŀ

 ��2013?��ͽ��ģ�⣩ijУѧ���������Ĺ�ҵ�����ռ�����ʳɷֽ����о���
��2013?��ͽ��ģ�⣩ijУѧ���������Ĺ�ҵ�����ռ�����ʳɷֽ����о���