��Ŀ����
����Ŀ��ij������ҵ�����������й©�¼������������ٱ������ר�Ҽ�ʱ��ѧ���ֳ��������¹ʵõ��˿��ơ�������Ƿ����������ˮ��Һ�������ԣ����о綾�Ժ�ʴ�ԣ���߱����ͨ�ԡ��������������й©ʱ������ɳ��������ʯ�ҵĻ��������Ӧ�����������з�Ӧ����Ч�ɷ�Ϊ��ʯ���е��������ơ������������Ϣ�����ش�
��1�������ʷ���ĽǶ���������������ʯ�Ҷ�����_____
��2�������¹�����35%�������4tй©����������������Ҫ�����������������ֳ�������_____
���𰸡������ 2.59t
��������
������Ƕ��ֳɷ���ɣ��������������Һ�ͺϽ�ȡ�����HF�������Ͷ�Ӧ�Ļ�ѧ����ʽ�������ĵ��������Ƶ�������
�⣺(1)������Ƿ����������ˮ��Һ������Ϊ������ʯ������Ҫ�ɷ�Ϊ�������ƣ��������������ʣ�����Ϊ����
(2)����Ҫ���������Ƶ�����Ϊx��
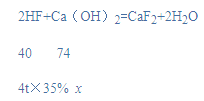
![]()
x=2.59t��
�𣺣�1�������ʷ���ĽǶ���������������ʯ�Ҷ����ڻ���
��2�������¹�����35%�������4tй©������������Ҫ2.59t�������������ֳ�������

 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�����Ŀ��ij��ѧ�С�����ѧϰ�������ƺ��������Ƶ����֪ʶ�������Ca��OH��2��NaOH���ܽ�����ݡ���ش��������⣺
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
�ܽ��/g | Ca��OH��2 | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 |
NaOH | 31 | 91 | 111 | 129 | 313 | 336 | |
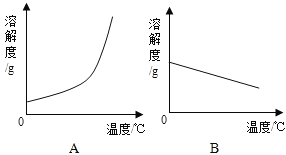
��1�������ϱ����ݣ�������Ca��OH��2��NaOH���ܽ�����ߣ�ͼ���ܱ�ʾNaOH�ܽ�����ߵ���_____���A����B������
��2����һƿ�ӽ����͵�Ca��OH��2��Һ��ɱ�����Һ�������ʩ�У�
�������������������������¶��������¶�������ˮ������ˮ���ٻָ���ԭ�¶�������������ʯ�ҡ�
���д�ʩ��ԭ������ȷ����_____������ĸ��
A �ڢܢ� B �ۢ� C �٢ڢݢ� D �٢ڢ�
��3��20��ʱ��191g����NaOH��Һ������10gˮ���ٻָ���20�棬������NaOH���塣��ʱ��Һ����������Ϊ_____
��4��20��ʱ�����ⶨNaOH��Һ��pH�����Ƚ�pH��ֽ������ˮ��ʪ���ٽ��вⶨ����������Һ��pH_____���ƫ��ƫС��������Ӱ�족����
��5���ɼ�������������Һ�ͳ���ʯ��ˮ��һ����ѧ��Ӧ����ʽΪ_____��