��Ŀ����
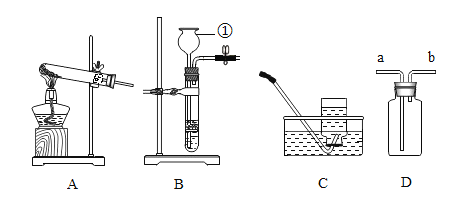
����Ŀ��ij�о���ѧϰС����������װ�ý����������ȡʵ�飬������ش���������:
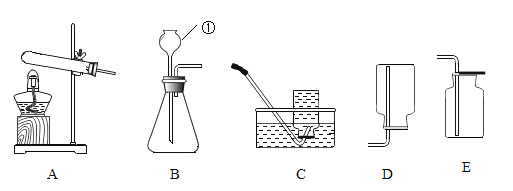
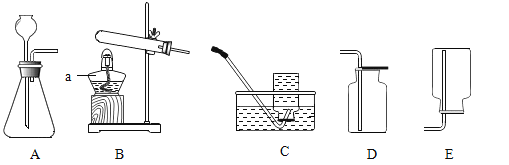
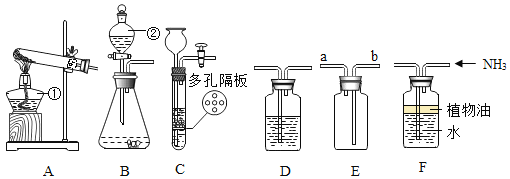
��1��д����Ţ�����������_________________________��
��2��ѡ��Aװ���ø��������ȡ�����Ļ�ѧ����ʽΪ_________________________���Թܿڷ���һС������Ŀ����_________________________; A�� C������ȡ��������Ҫ�Ƚ������Ƴ�ˮ�棬��Ϩ��ƾ��ƣ�ԭ����_________________________��
��3��ѡ��Bװ�ã���п����ϡ������ȡ�����Ļ�ѧ����ʽΪ_________________________��Ϊ�˿��Ƹ÷�Ӧ�ķ�����ֹͣ����Ӧ�ò������Ʒ��_________________________��
��4������Dװ���ռ�������̼��������Ӧ��_________________________��ͨ�루����a������b����������Dװ�ü����Ƶõ������Ƿ�Ϊ������̼������Dװ���з���һ������_________________________ ����һ�����ʵ����ƣ���������Ӧ�Ļ�ѧ����ʽΪ_________________________��
���𰸡�����©�� 2KMnO4![]() K2MnO4+MnO2+O2�� ��ֹ������ؿ����������� ��ֹˮ����ʹ�Թ�ը�� Zn+ H2SO4 = ZnSO4 + H2�� ������ a ����ʯ��ˮ CO2 + Ca(OH)2 = CaCO3��+ H2O
K2MnO4+MnO2+O2�� ��ֹ������ؿ����������� ��ֹˮ����ʹ�Թ�ը�� Zn+ H2SO4 = ZnSO4 + H2�� ������ a ����ʯ��ˮ CO2 + Ca(OH)2 = CaCO3��+ H2O
��������
��1����Ţ����������Ƴ���©����
��2�����������ȡ������ԭ���Ǹ�������ڼ��������·ֽ���������ء��������̡���������ѧ����ʽΪ2KMnO4![]() K2MnO4+MnO2+O2�����Թܿڷ���һС������Ŀ���Ƿ�ֹ������ؿ����������ܣ� A�� C������ȡ��������Ҫ�Ƚ������Ƴ�ˮ�棬��Ϩ��ƾ��ƣ�ԭ���Ƿ�ֹˮ����ʹ�Թ�ը�ѣ�
K2MnO4+MnO2+O2�����Թܿڷ���һС������Ŀ���Ƿ�ֹ������ؿ����������ܣ� A�� C������ȡ��������Ҫ�Ƚ������Ƴ�ˮ�棬��Ϩ��ƾ��ƣ�ԭ���Ƿ�ֹˮ����ʹ�Թ�ը�ѣ�
��3����п����ϡ������ȡ������ԭ����п�����ᷴӦ��������п����������ѧ����ʽΪZn+ H2SO4 = ZnSO4 + H2����Ϊ�˿��Ƹ÷�Ӧ�ķ�����ֹͣ����Ӧ�ò������Ʒ���ܷ��ڴ��Թ��еĴ����壬ԭ���ǣ��رյ��ɼУ���������ѹǿ�����Թ���Һ���½����½��������·������Һ����룬��Ӧֹͣ�����ɼУ�������٣�ѹǿ��С��©����Һ���½����Թ���Һ����������������Һ��Ӵ�ʱ����Ӧ��ʼ��
��4������Dװ���ռ�������̼��������̼���ܶȱȿ����Ĵ�������Ӧ��a��ͨ�롣����Dװ�ü����Ƶõ������Ƿ�Ϊ������̼������Dװ���з���һ�����ij���ʯ��ˮ��ʯ��ˮ����ǿ�֤�������Ƕ�����̼��������Ӧ�Ļ�ѧ����ʽΪCO2 + Ca(OH)2 = CaCO3��+ H2O��

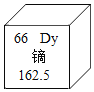
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�