��Ŀ����
������ʯ�����Ʋ�Ʒ֮һ������������Ҫȼ�ϣ���ش���1�����ͳ��ȼ�յIJ����Ƕ�����̼��ˮ���ɴ˿��ƶ����͵����Ԫ��һ������ �� Ԫ�أ�
��2��������Ϊȼ�ϵ�����β������һ����̼����������ȴ�����Ⱦ�Ŀǰ����������β����Ⱦ�Ĵ�ʩ֮һ�����������м����������Ҵ�����ѧʽΪC2H5OH�����Ҵ��������� ��ѡ��������������д���Ҵ��ڿ�������ȫȼ�յĻ�ѧ��Ӧ����ʽ�� ��
��3��������������͵�ȼ���У�ȼ�ղ����������� �����ţ���
����Ȼ�� ���Ҵ� ������
��4����ѧ��Ԥ�ԣ�δ���������ȼ����Դ����ɫֲ���ɫֲ���������з���������ת���� ��ѡ����ţ�
A����̫����ת��Ϊ��ѧ�� B��������ת��Ϊ���ܣ�
���𰸡���������1�����������غ㶨�ɿ��ǣ���2��������������ֻ���������������ɣ����ݷ���ʽ��д�����ǣ���3�����������������ŵ㿼�ǣ���4�����ݹ�����õIJ���ǣ�
����⣺��1����Ӧǰ��Ԫ������䣬����������ȼ�����ɶ�����̼��ˮ��˵��������һ������̼Ԫ�غ���Ԫ�أ����ܺ�����Ԫ�أ�
��2���Ҵ������к����Ҵ������ͣ����ڻ����Ҵ��ڿ�������ȫȼ�յķ�Ӧ�����Ҵ����������������Ƕ�����̼��ˮ������ƽ̼���⣬�����ƽ������Ӧ�����ǵ�ȼ��
��3����ȡ��������Դ�ḻ������ȼ�շų��������࣬����ȼ�ղ�����ˮ������Ⱦ����������������������Դ��
��4����ɫֲ�������õIJ����ǵ��ۺ����������Է���������ת�����ɹ��ܱ�Ϊ��ѧ�ܣ�
�ʴ�Ϊ����1��̼���⣻��2������C2H5OH+3O2 2CO2+3H2O����3���ۣ���4��A��
2CO2+3H2O����3���ۣ���4��A��
�����������ؼ���Ҫ֪�������غ㶨�����ݣ�����������ý��ʵ�����⣬��Ϥ����ʽ����д������
����⣺��1����Ӧǰ��Ԫ������䣬����������ȼ�����ɶ�����̼��ˮ��˵��������һ������̼Ԫ�غ���Ԫ�أ����ܺ�����Ԫ�أ�
��2���Ҵ������к����Ҵ������ͣ����ڻ����Ҵ��ڿ�������ȫȼ�յķ�Ӧ�����Ҵ����������������Ƕ�����̼��ˮ������ƽ̼���⣬�����ƽ������Ӧ�����ǵ�ȼ��
��3����ȡ��������Դ�ḻ������ȼ�շų��������࣬����ȼ�ղ�����ˮ������Ⱦ����������������������Դ��
��4����ɫֲ�������õIJ����ǵ��ۺ����������Է���������ת�����ɹ��ܱ�Ϊ��ѧ�ܣ�
�ʴ�Ϊ����1��̼���⣻��2������C2H5OH+3O2
 2CO2+3H2O����3���ۣ���4��A��
2CO2+3H2O����3���ۣ���4��A�������������ؼ���Ҫ֪�������غ㶨�����ݣ�����������ý��ʵ�����⣬��Ϥ����ʽ����д������

��ϰ��ϵ�д�
�����Ŀ
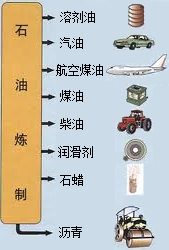 �й�ʯ����Ȼ�����Ź�˾�����������ڲ�����̲���������ִ�����ģ��ʮ�ڶֵĴ�����--�����ϱ���������ǿ�ҹ���Դ��ȫ��Ӧ�ı�������������Ҫ���壮ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã���ͼ����
�й�ʯ����Ȼ�����Ź�˾�����������ڲ�����̲���������ִ�����ģ��ʮ�ڶֵĴ�����--�����ϱ���������ǿ�ҹ���Դ��ȫ��Ӧ�ı�������������Ҫ���壮ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã���ͼ������1��ʯ������
��2��������ʯ�����ƵIJ�Ʒ֮һ��ֱ����Ϊȼ��ʹ������ɻ�����Ⱦ���������м��������Ҵ����ɽ�ʡʯ����Դ����������β����Ⱦ��д���Ҵ��ڿ�����ȼ�յĻ�ѧ����ʽ��
��3��ʯ�����ƵIJ�Ʒ֮һʯ����������������
Ϊ�˲ⶨ������̼��������Ԫ�ص������ȣ�ij��ѧ��ȤС�����������ͼ��ʾ��ʵ�飮ʵ�鲽�����£��ȷֱ��������װ�â�װ�â����������ͼʾ���Ӻ�����װ�ã���ȼ����ͬʱ��a���ܿڳ�����һ��ʱ���Ϩ�������ٷֱ��������װ�â�װ�â��������ʵ�����ݼ��±��������ԭ��������H-1 C-12 O-16��
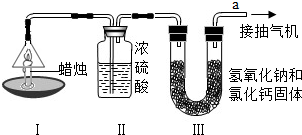
| ���� | װ�â� | װ�â� | |
| ��Ӧǰ������/g | 15.8 | 182.3 | 212.2 |
| ��Ӧ�������/g | 14.4 | 184.1 | 216.6 |
���ɸ�ʵ�����ݼ��㣬������̼����Ԫ�ص�����֮��Ϊ
��װ�â��װ�â��˳���ܷ�ߵ���
�������ϣ�װ�â��װ�â����ӵ���������������ʧȥ����������ԭ����
�ݸ�ʵ���ܷ�ȷ�������ȼ�����ɶ�����̼��ˮ��������
| |||||||||||||||
 �й�ʯ����Ȼ�����Ź�˾�����������ڲ�����̲���������ִ�����ģ��ʮ�ڶֵĴ�����--�����ϱ���������ǿ�ҹ���Դ��ȫ��Ӧ�ı�������������Ҫ���壮ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã���ͼ����
�й�ʯ����Ȼ�����Ź�˾�����������ڲ�����̲���������ִ�����ģ��ʮ�ڶֵĴ�����--�����ϱ���������ǿ�ҹ���Դ��ȫ��Ӧ�ı�������������Ҫ���壮ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã���ͼ����
��1��ʯ������________�ѡ���ϡ���������ʯ�ͷ�������________��ѡ����������ѧ���仯��
��2��������ʯ�����ƵIJ�Ʒ֮һ��ֱ����Ϊȼ��ʹ������ɻ�����Ⱦ���������м��������Ҵ����ɽ�ʡʯ����Դ����������β����Ⱦ��д���Ҵ��ڿ�����ȼ�յĻ�ѧ����ʽ��________��
��3��ʯ�����ƵIJ�Ʒ֮һʯ����������������
Ϊ�˲ⶨ������̼��������Ԫ�ص������ȣ�ij��ѧ��ȤС�����������ͼ��ʾ��ʵ�飮ʵ�鲽�����£��ȷֱ��������װ�â�װ�â����������ͼʾ���Ӻ�����װ�ã���ȼ����ͬʱ��a���ܿڳ�����һ��ʱ���Ϩ�������ٷֱ��������װ�â�װ�â��������ʵ�����ݼ��±��������ԭ��������H-1��C-12��O-16��

| ���� | װ�â� | װ�â� | |
| ��Ӧǰ������/g | 15.8 | 182.3 | 212.2 |
| ��Ӧ�������/g | 14.4 | 184.1 | 216.6 |
���ɸ�ʵ�����ݼ��㣬������̼����Ԫ�ص�����֮��Ϊ________��
��װ�â��װ�â��˳���ܷ�ߵ���________����ܡ����ܡ�����
�������ϣ�װ�â��װ�â����ӵ���������������ʧȥ����������ԭ����________��
�ݸ�ʵ���ܷ�ȷ�������ȼ�����ɶ�����̼��ˮ��������________����ܡ����ܡ�����������________��