��Ŀ����
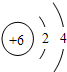 18��̼��̼�Ļ������ڹ�ũҵ�������ճ��������й㷺����;����ش�
18��̼��̼�Ļ������ڹ�ũҵ�������ճ��������й㷺����;����ش���1����ͼ��̼ԭ�ӽṹʾ��ͼ��̼ԭ�ӵ���������
6
���������4
���ӣ���2���Ŵ���ī��д���Ƶ��ֻ�������Բ���ɫ��ԭ����ī����Ҫ�ɷ�̼�ڳ����±Ƚ�
������/�ȶ�
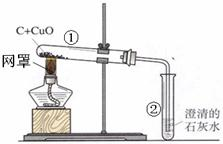
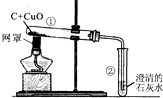
����3��������̼�ܲ�����������ɴ����еġ�̼ѭ���������Ǵ����ж�����̼�ĺ��������������ᵼ��_
����ЧӦ
���Ӷ�ʹȫ���ů���߲˴�������������ϵĶ�����̼�����ö��ַ����Ƶã����������ϡ���ᣨH2SO4����̼����泥�NH4HCO3����Ӧ�Ƶã���Ӧ����������李�ˮ�Ͷ�����̼����һ��Ӧ�Ļ�ѧ����ʽ��H2SO4+2NH4HCO3�T��NH4��2SO4+2H2O+2CO2��
����4���о����֣����ʵĽṹ�������ʣ����ʵ����ʾ�����;��ʯī�ͽ��ʯ����̼��һ�ֵ��ʣ��������ʲ�ͬ������
̼ԭ�ӵ����з�ʽ
��ͬ����������1����̼ԭ�ӽṹʾ��ͼ��֪��̼ԭ�ӵ�ԭ�Ӻ�����6�����ӣ��������4�����ӣ�
��2��̼�Ļ�ѧ������ͨ������±Ƚ��ȶ������������������ʷ�����ѧ��Ӧ��������̼��һ����Ҫ���������壬�����ŷſ�����������ЧӦ
��3��������̼�����������γ�������ЧӦ��̼��������ų����������̼��̼����������ᷴӦ��������李�ˮ�Ͷ�����̼��
��4�����ʵĽṹ�������ʵ����ʣ����ʯ��ʯī������̼ԭ��ֱ�ӹ��ɵģ���̼ԭ�ӵ����з�ʽ��ͬ�������������ʲ���ͬ
��2��̼�Ļ�ѧ������ͨ������±Ƚ��ȶ������������������ʷ�����ѧ��Ӧ��������̼��һ����Ҫ���������壬�����ŷſ�����������ЧӦ
��3��������̼�����������γ�������ЧӦ��̼��������ų����������̼��̼����������ᷴӦ��������李�ˮ�Ͷ�����̼��
��4�����ʵĽṹ�������ʵ����ʣ����ʯ��ʯī������̼ԭ��ֱ�ӹ��ɵģ���̼ԭ�ӵ����з�ʽ��ͬ�������������ʲ���ͬ
����⣺��1������̼ԭ�ӽṹʾ��ͼ��֪��̼ԭ�ӵ���������6���������4�����ӣ����6��4��
��2���Ŵ���ī��д����Ƶ��ֻ�������Բ���ɫ����ԭ����ī�е���Ҫ�ɷ�̼�����ȶ��ԣ�����ȶ���
��3��������̼�ܲ�����������ɴ����еġ�̼ѭ���������Ǵ����ж�����̼�ĺ���������������ʹȫ�����±�ů���Ӷ���������ЧӦ��ϡ���ᣨH2SO4����̼����泥�NH4HCO3����Ӧ��������李�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪH2SO4+2NH4HCO3�T��NH4��2SO4+2H2O+2CO2�� �������ЧӦ��H2SO4+2NH4HCO3�T��NH4��2SO4+2H2O+2CO2��
��4�����ʵĽṹ�������ʵ����ʣ����ʯ��ʯī������̼ԭ��ֱ�ӹ��ɵģ���̼ԭ�ӵ����з�ʽ��ͬ�������������ʲ���ͬ���ʴ�Ϊ��̼ԭ�ӵ����з�ʽ
��2���Ŵ���ī��д����Ƶ��ֻ�������Բ���ɫ����ԭ����ī�е���Ҫ�ɷ�̼�����ȶ��ԣ�����ȶ���
��3��������̼�ܲ�����������ɴ����еġ�̼ѭ���������Ǵ����ж�����̼�ĺ���������������ʹȫ�����±�ů���Ӷ���������ЧӦ��ϡ���ᣨH2SO4����̼����泥�NH4HCO3����Ӧ��������李�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪH2SO4+2NH4HCO3�T��NH4��2SO4+2H2O+2CO2�� �������ЧӦ��H2SO4+2NH4HCO3�T��NH4��2SO4+2H2O+2CO2��
��4�����ʵĽṹ�������ʵ����ʣ����ʯ��ʯī������̼ԭ��ֱ�ӹ��ɵģ���̼ԭ�ӵ����з�ʽ��ͬ�������������ʲ���ͬ���ʴ�Ϊ��̼ԭ�ӵ����з�ʽ
������������Ҫ����ԭ�ӽṹ��̼�Ļ�ѧ���ʵȷ����֪ʶ�����ʱӦ������ԭ�ӽṹʾ��ͼ�ĺ��壬Ҫ����̼�������ر���ͨ������»�ѧ�����ȶ������ݣ�

��ϰ��ϵ�д�
 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
�����Ŀ
 17��̼��̼�Ļ������ڹ�ũҵ�������ճ��������й㷺����;��
17��̼��̼�Ļ������ڹ�ũҵ�������ճ��������й㷺����;��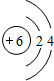 ̼��̼�Ļ������ڹ�ũҵ�������ճ��������й㷺����;��
̼��̼�Ļ������ڹ�ũҵ�������ճ��������й㷺����;��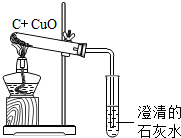 ��3��������̼�ܲ�����������ɴ����еġ�̼ѭ����������
��3��������̼�ܲ�����������ɴ����еġ�̼ѭ����������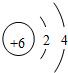
 ̼��̼�Ļ������ڹ�ũҵ�������ճ��������й㷺����;��
̼��̼�Ļ������ڹ�ũҵ�������ճ��������й㷺����;��