��Ŀ����
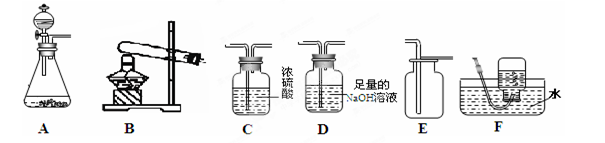
���������ʵ��װ��ͼ�ش����⡣

��1��д������a��b�����ƣ�a�� ��b�� ��
��2������Bװ����ȡ��������Ӧ�Ļ�ѧ����ʽ�� ��
��3��ʵ�����ü����Ȼ�狀��������ƹ�������ķ�����ȡ������NH3����ͬʱ�õ��� ���ƺ�ˮ���÷�Ӧ�Ļ�ѧ����ʽ�� ��Ӧѡ�õķ���װ��Ϊ ����װ�ñ�ţ���
��4���ռ�����ʱӦѡ��Dװ�ã����ռ��������ļ���ƿ�����ڵ�����ɫ��̪��Һ��
ˮ���У��۲쵽����ƿ���д�����ɫҺ����롣����������Ϣ�ܽ������������
�� �� ���ش��������ɣ���

��1��д������a��b�����ƣ�a�� ��b�� ��
��2������Bװ����ȡ��������Ӧ�Ļ�ѧ����ʽ�� ��
��3��ʵ�����ü����Ȼ�狀��������ƹ�������ķ�����ȡ������NH3����ͬʱ�õ��� ���ƺ�ˮ���÷�Ӧ�Ļ�ѧ����ʽ�� ��Ӧѡ�õķ���װ��Ϊ ����װ�ñ�ţ���
��4���ռ�����ʱӦѡ��Dװ�ã����ռ��������ļ���ƿ�����ڵ�����ɫ��̪��Һ��
ˮ���У��۲쵽����ƿ���д�����ɫҺ����롣����������Ϣ�ܽ������������
�� �� ���ش��������ɣ���
��1���Թ� ��Һ©��
��2��2H2O2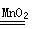 2H2O+O2��
2H2O+O2��
��3��2NH4Cl+Ca��OH��2 CaCl2+2NH3��+2H2O A
CaCl2+2NH3��+2H2O A
��4���������ܶȱȿ���С ������������ˮ�γɰ�ˮ
��2��2H2O2
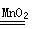 2H2O+O2��
2H2O+O2����3��2NH4Cl+Ca��OH��2
 CaCl2+2NH3��+2H2O A
CaCl2+2NH3��+2H2O A��4���������ܶȱȿ���С ������������ˮ�γɰ�ˮ
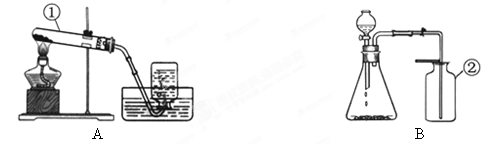
�����������1��������������ͼ��a���Թܣ�b�Ƿ�Һ©�����ʴ�Ϊ���Թܣ���Һ©����
��2��Bװ�������ڹ����Һ��IJ����ȷ�Ӧ����ȡ����ʱ���ö��������������ֽ����������Һ����ѧ��Ӧ����ʽ�ǣ�2H2O2
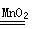 2H2O+O2����
2H2O+O2������3���Ƶð������ü����Ȼ�狀��������ƹ�������ķ��������ڡ���������͡�������ѡ��A����ѧ��Ӧ����ʽ�ǣ�2NH4Cl+Ca��OH��2
 CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O����4���ռ�����ʱӦѡ��Dװ�ã�˵���������ܶ�С�ڿ������ܶȣ���ˮ�Լ��ԣ�������Һ��ʹ��̪��Һ��죬����ƿ���д�����ɫҺ����룬˵��������������ˮ�γɰ�ˮ���ʴ�Ϊ���������ܶȱȿ���С��������������ˮ�γɰ�ˮ��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ