��Ŀ����
CuSO4��Һ�Թ�������ķֽ���д����ã���ͬѧ������������ҺҲ���������Ӧ����ͬ�������ã����ǽ��������µ�ʵ��̽����
��1��������������ʵ�鱨�棺
��2����֪FeCl3��ˮ�пɽ����Fe3+��Cl-�������ܹ���H2O2�ķֽ�������õijɷ֣���λͬѧ����˲��룺�IJ�����H2O���ҵIJ�����Fe3+�����IJ�����Cl-������Ϊ����ܵ����ĸ�ͬѧ�IJ��룬Ϊʲô��
��3�������µ��������룬ͬѧ����ʵ�������̽����������ϸ�����������
��4����ͬѧ���顰����ʢ��5mL5%��H2O2��Һ���Թ��м�������Fe2��SO4��3��Һ���Խ�һ��ȷ��Fe3+�Ĵ����á�����ʵ��������ޱ�Ҫ����˵�����ɣ�
��1��������������ʵ�鱨�棺
| ʵ����� | ʵ������ | ʵ����� |
| ��һ֧�Թ��м���5mL5%��H2O2��Һ��Ȼ�����������FeCl3��Һ���Ѵ����ǵ�ľ�������Թܣ� | ______ | FeCl3��Һ���Զ�H2O2�ķֽ�������� |
��3�������µ��������룬ͬѧ����ʵ�������̽����������ϸ�����������
| ʵ����� | ʵ������ | ���� |
| ����ʢ��5mL5%��H2O2��Һ���Թ��м���������ϡ���ᣬ���Ѵ����ǵ�ľ�������Թܣ� | ���������� | ______ |
��1�����������ô����ǵ�ľ�����飬�����һ֧�Թ��м���5mL5%��H2O2��Һ��Ȼ�����������FeCl3��Һ���Ѵ����ǵ�ľ�������Թܣ��Թ��п��ٲ����������ݣ���ʹ�����ǵ�ľ����ȼ�����ۣ�FeCl3��Һ���Զ�H2O2�ķֽ�������ã��ʴ�Ϊ���Թ��п��ٲ����������ݣ���ʹ�����ǵ�ľ����ȼ��
��2����FeCl3��Һ������������H2O��Fe3+��Cl-������Ϊ����ܵ��Ǽ�ͬѧ�IJ��룻��ΪH2O2��Һ�к��д���ˮ����H2O2��û�п��ٷֽ⣮�ʴ�Ϊ����ͬѧ�IJ��룻��ΪH2O2��Һ�к��д���ˮ����H2O2��û�п��ٷֽ⣮
��3����ʢ��5mL5%��H2O2��Һ���Թ��м���������ϡ���ᣬ���Ѵ����ǵ�ľ�������Թܣ������������ܴ�H2O2�ֽ�IJ���Cl-��H+���ʴ�Ϊ���ܴ�H2O2�ֽ�IJ���Cl-��H+��
��4��û�б�Ҫ��һ��ȷ��Fe3+�Ĵ����ã� ��ΪFeCl3��Һ��������Fe3+��Cl-��H2O��H+��ǰ��ʵ����ƶ��Ѿ�֤��Cl-��H2O��H+��H2O2�ķֽ������ã�˵��ֻ��Fe3+��H2O2�ķֽ��д����ã��ʴ�Ϊ��û�б�Ҫ�� ��ΪFeCl3��Һ��������Fe3+��Cl-��H2O��H+��ǰ��ʵ����ƶ��Ѿ�֤��Cl-��H2O��H+��H2O2�ķֽ������ã�˵��ֻ��Fe3+��H2O2�ķֽ��д����ã�
��2����FeCl3��Һ������������H2O��Fe3+��Cl-������Ϊ����ܵ��Ǽ�ͬѧ�IJ��룻��ΪH2O2��Һ�к��д���ˮ����H2O2��û�п��ٷֽ⣮�ʴ�Ϊ����ͬѧ�IJ��룻��ΪH2O2��Һ�к��д���ˮ����H2O2��û�п��ٷֽ⣮
��3����ʢ��5mL5%��H2O2��Һ���Թ��м���������ϡ���ᣬ���Ѵ����ǵ�ľ�������Թܣ������������ܴ�H2O2�ֽ�IJ���Cl-��H+���ʴ�Ϊ���ܴ�H2O2�ֽ�IJ���Cl-��H+��
��4��û�б�Ҫ��һ��ȷ��Fe3+�Ĵ����ã� ��ΪFeCl3��Һ��������Fe3+��Cl-��H2O��H+��ǰ��ʵ����ƶ��Ѿ�֤��Cl-��H2O��H+��H2O2�ķֽ������ã�˵��ֻ��Fe3+��H2O2�ķֽ��д����ã��ʴ�Ϊ��û�б�Ҫ�� ��ΪFeCl3��Һ��������Fe3+��Cl-��H2O��H+��ǰ��ʵ����ƶ��Ѿ�֤��Cl-��H2O��H+��H2O2�ķֽ������ã�˵��ֻ��Fe3+��H2O2�ķֽ��д����ã�

��ϰ��ϵ�д�
�����Ŀ
 ˮ����Ҫ����Ȼ��Դ���ڽ��л�ѧʵ�����Һ�ж���Ҫˮ���밴Ҫ��ش��������⣺
ˮ����Ҫ����Ȼ��Դ���ڽ��л�ѧʵ�����Һ�ж���Ҫˮ���밴Ҫ��ش��������⣺
��1�����С�ˮ�������ڴ��������______��
A����Ȫˮ�� B����ˮ����C������ˮ����D������ˮ
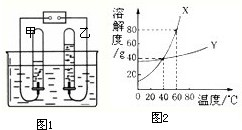
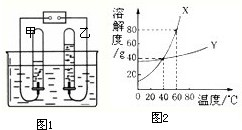
��2����ͼ1�ǵ��ˮ��ʵ��װ�ã����Թ����ռ�����������______��
��3���ҹ���������ˮ����Ҫ��Ϊ��
| �й�ָ�� | ��ѧָ�� | ϸ��ָ�� |
| ˮ����ɫ��ζ���� | pH��6.5��8.5 | ϸ���ܸ�����100��/mL�� |
��4��ˮ�������ܼ��������������ù���������Һ�Ի�������������������������Ϊ15%�Ĺ���������Һ10g��Ҫ���Ƴ�������������Ϊ1%�Ĺ���������Һ�����ˮ______g��
��5��ͼ2��X��Y���ֹ���������ˮ�е��ܽ������ͼ��
��60��ʱ����45g��X����50gˮ�У�����ܽ⣬�õ����¶���X��ˮ��Һ��______������͡������͡�����Һ��
����40��ʱ���ֱ�100gX�ı�����Һ��200gY�ı�����Һ������10gˮ����������������ֱ�Ϊmg��ng����m______n�����������=����������
��6����ˮ����������һ�����ɣ���������ɻ�ʹѧϰ�����ɣ��������ְ�ɫ���壬�ֱ���NaOH��CuSO4��NaCl��CaCO3��ֻ�ṩ����ˮ���������
�ٸ����Ƿ�����ˮ������ˮʱ�γ���ɫ��Һ���ɷֱ�����______��
�ڽ�����ˮʱ�γɵ���ɫ��Һ���������ʷ�Ϊһ�飬������Һ�¶�______������ߡ��������͡����䡱�����ɼ����______��
����Уʵ����ʵʩ��������2011���п���ѧʵ���������ո������Ļ��ʵ�鿼����H2O2�ֽ�������Ϊ�β�����MnO2��������ͬѧ��Ϊ֮չ����̽����
I��������������ʵ�������50mL��6%��H2O2��Һ��
[������Һ]
����һƿ�¹��õ�˫��ˮ����ǩ��ͼ��ʾ��
��1��������ƿ˫��ˮ������6%��ҽ��˫��ˮ1500g����Ҫ����˫��ˮ�������Ƕ��ٿˣ�
��2������һƿ5����ǰ��������ͬ����˫��ˮ������⣬�ѻ����ֽ�ų���9.6g������������ʣ��ĸ�ƿ��Һ�����ʵ�����������
II��Ȼ��ͬѧ���ֱȽ��˸������������¶�50mL��6%��H2O2��Һ�ֽⷴӦ�Ĵ����������
[ʵ���¼]��������50mL��6%��H2O2��Һ�ֽⷴӦ�Ĵ��������������22�棩
[ʵ�����]�ɱ������ݼ�ʵ��������Եó���MnO2 ���ֽ�H2O2�����������㲻�㣬�ٿ�ʼ��Ӧ���ʹ��죬������������װ����ѹǿ��Ȼ����ʹ�÷�Һ©����Һ�������裬�ռ�������ˮ���ſ���ʱ�����飬�ͻ��в���O2 ��ʧ�����ռ����һƿO2��Ӧ��������ʱ̫������ ��
[�������]ͬѧ����Ϊ��FeCl3��Һ������Ч����ã���FeCl3��ҺΪ���ܴ��ֽ�H2O2��
[ʵ�����]��֪FeCl3��ˮ�пɽ����Fe3+ ��Cl-��ͬѧ��������²��룺
��ͬѧ�IJ����ǣ��������ֽ�H2O2����FeCl3��Һ�е�H2O��
��ͬѧ�IJ����ǣ��������ֽ�H2O2����FeCl3��Һ�е�Fe3+��
��ͬѧ�IJ����ǣ��������ֽ�H2O2����FeCl3��Һ�е�Cl-��
����Ϊ����ܵ��� ͬѧ�IJ��룬������ ��
ͬѧ�Ƕ����µ��������룬��ʵ�������̽����������ϸ��������գ�
[ʵ�����1]��ʢ��5mL��6% H2O2��Һ���Թ��м���������HCl��Һ�����Ѵ����ǵ�ľ�������Թܣ�
[ʵ������1]����������Ӧ���ۣ� ��
[ʵ�����2]����ʢ��5mL��6%��H2O2��Һ���Թ��м��������� �����ٴΰѴ����ǵ�ľ�������Թܣ�
[ʵ������2] ��
��Ӧ���ۣ� ��
[��չ�о�]
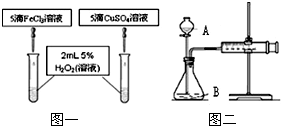
ͬѧ�ǻ��Ƚ���FeCl3��CuSO4�����ζ�H2O2�ֽ�Ĵ�Ч�������Ƿֳɼס������飬�ֱ��������ͼһ��ͼ����ʾ��ʵ�鲢������ʵ�飮������������Һ��Ũ�ȵ�������ͬ��

��1��ָ���������ƣ�A B
��2��д����FeCl3Ϊ�����ķ�Ӧ�Ļ�ѧ����ʽ ��
��3��ͼһ��ͨ���۲� �����ԱȽϵó����ۣ���ͬѧ�����ҩƷCuSO4��ΪCuCl2��Ϊ�������������� ������ΪҩƷ���������θĽ��� ��
��4�����ͼ��װ�������Եķ����ǣ� ������رա�������A�Ļ�������ע������������һ�����룬һ��ʱ����ɿ��������۲쵽�����ֻص���ԭλ����˵��װ�������� ������á����á���������ͬѧ����ͼ����ʾʵ��������������� ������ţ�A��һ��ʱ�����ռ������������ B���ռ�һ���������������˫��ˮ����������
�о�С�黹����ҺŨ�ȡ��¶ȵ�ʵ������������̽�����±�ѡȡ�˲���ʵ�����ݣ�
�����������H2O2��Һ��ȡ��ͬ���O2�����ʱ��
[��������]���ϱ��л��ܵó��Ľ����ǣ� ��

I��������������ʵ�������50mL��6%��H2O2��Һ��
[������Һ]
����һƿ�¹��õ�˫��ˮ����ǩ��ͼ��ʾ��
��1��������ƿ˫��ˮ������6%��ҽ��˫��ˮ1500g����Ҫ����˫��ˮ�������Ƕ��ٿˣ�
��2������һƿ5����ǰ��������ͬ����˫��ˮ������⣬�ѻ����ֽ�ų���9.6g������������ʣ��ĸ�ƿ��Һ�����ʵ�����������
II��Ȼ��ͬѧ���ֱȽ��˸������������¶�50mL��6%��H2O2��Һ�ֽⷴӦ�Ĵ����������
[ʵ���¼]��������50mL��6%��H2O2��Һ�ֽⷴӦ�Ĵ��������������22�棩
| ���������� | O2 ƽ������ | ��������ʱ�� | �ɱ� | ��Ӧ��� |
| MnO21.0g | 919mL | 10��03�� | 0.11Ԫ | �ֽ��ȿ���� |
| 10%FeCl3��Һ5�� | 985mL | 4��50�� | 0.02Ԫ | ���Ȳ���O2 |
| 15%CuSO4��Һ5�� | 955mL | 4��57�� | 0.03Ԫ | ���Ȳ���O2 |
[�������]ͬѧ����Ϊ��FeCl3��Һ������Ч����ã���FeCl3��ҺΪ���ܴ��ֽ�H2O2��
[ʵ�����]��֪FeCl3��ˮ�пɽ����Fe3+ ��Cl-��ͬѧ��������²��룺
��ͬѧ�IJ����ǣ��������ֽ�H2O2����FeCl3��Һ�е�H2O��
��ͬѧ�IJ����ǣ��������ֽ�H2O2����FeCl3��Һ�е�Fe3+��
��ͬѧ�IJ����ǣ��������ֽ�H2O2����FeCl3��Һ�е�Cl-��
����Ϊ����ܵ��� ͬѧ�IJ��룬������ ��
ͬѧ�Ƕ����µ��������룬��ʵ�������̽����������ϸ��������գ�
[ʵ�����1]��ʢ��5mL��6% H2O2��Һ���Թ��м���������HCl��Һ�����Ѵ����ǵ�ľ�������Թܣ�
[ʵ������1]����������Ӧ���ۣ� ��
[ʵ�����2]����ʢ��5mL��6%��H2O2��Һ���Թ��м��������� �����ٴΰѴ����ǵ�ľ�������Թܣ�
[ʵ������2] ��
��Ӧ���ۣ� ��
[��չ�о�]
ͬѧ�ǻ��Ƚ���FeCl3��CuSO4�����ζ�H2O2�ֽ�Ĵ�Ч�������Ƿֳɼס������飬�ֱ��������ͼһ��ͼ����ʾ��ʵ�鲢������ʵ�飮������������Һ��Ũ�ȵ�������ͬ��

��1��ָ���������ƣ�A B
��2��д����FeCl3Ϊ�����ķ�Ӧ�Ļ�ѧ����ʽ ��
��3��ͼһ��ͨ���۲� �����ԱȽϵó����ۣ���ͬѧ�����ҩƷCuSO4��ΪCuCl2��Ϊ�������������� ������ΪҩƷ���������θĽ��� ��
��4�����ͼ��װ�������Եķ����ǣ� ������رա�������A�Ļ�������ע������������һ�����룬һ��ʱ����ɿ��������۲쵽�����ֻص���ԭλ����˵��װ�������� ������á����á���������ͬѧ����ͼ����ʾʵ��������������� ������ţ�A��һ��ʱ�����ռ������������ B���ռ�һ���������������˫��ˮ����������
�о�С�黹����ҺŨ�ȡ��¶ȵ�ʵ������������̽�����±�ѡȡ�˲���ʵ�����ݣ�
�����������H2O2��Һ��ȡ��ͬ���O2�����ʱ��
| Ũ�� ʱ�䣨min�� ���� | 30% H2O2 | 15% H2O2 | 5% H2O2 |
| ����a g MnO2 | 0.2 | 0.8 | 2.0 |

 ˮ����Ҫ����Ȼ��Դ���ڽ��л�ѧʵ�����Һ�ж���Ҫˮ���밴Ҫ��ش��������⣺
ˮ����Ҫ����Ȼ��Դ���ڽ��л�ѧʵ�����Һ�ж���Ҫˮ���밴Ҫ��ش��������⣺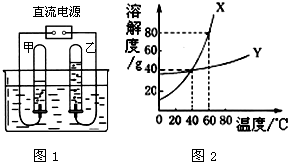 ˮ����Ҫ����Ȼ��Դ���ڽ��л�ѧʵ�����Һ�ж���Ҫˮ���밴Ҫ��ش��������⣺
ˮ����Ҫ����Ȼ��Դ���ڽ��л�ѧʵ�����Һ�ж���Ҫˮ���밴Ҫ��ش��������⣺ ����Уʵ����ʵʩ��������2011���п���ѧʵ���������ո������Ļ��ʵ�鿼����H2O2�ֽ�������Ϊ�β�����MnO2��������ͬѧ��Ϊ֮չ����̽����
����Уʵ����ʵʩ��������2011���п���ѧʵ���������ո������Ļ��ʵ�鿼����H2O2�ֽ�������Ϊ�β�����MnO2��������ͬѧ��Ϊ֮չ����̽����