��Ŀ����
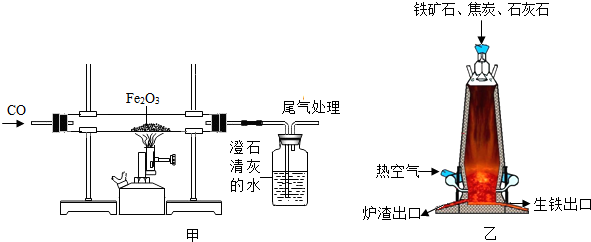
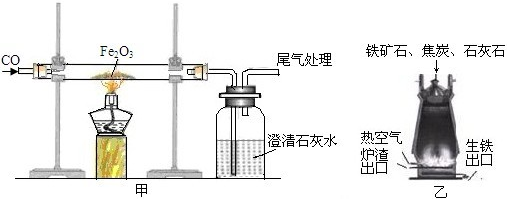
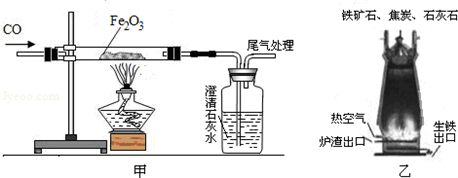
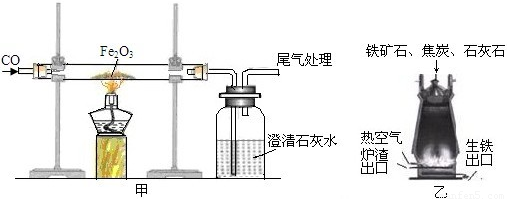
��ͼ��һ����̼����������Ӧװ�õ�ʾ��ͼ����ͼ�Ǹ�¯����ʾ��ͼ����ش��������⡣
(1)д����ͼ�е�ʵ������__________________________________________________
________________________________________________________________________��
(2)��ͼ��װ�õIJ���֮����________________________________________________
________________________________________________________________________��
(3)��¯����ԭ���н�̿��������____________________________________________
________________________________________________________________________��
(4)��д����¯���������Ļ�ѧ����ʽ________________________________________
________________________________________________________________________��

��

��
(5)���к����ʵ���������Ʒ10 g(���ʲ��μӷ�Ӧ)��Ϊ�ⶨ����Ʒ��������������������ijͬѧ�ø�����ļ�ͼװ�ý���ʵ�飬�õ������������ݣ�
| ��Ӧǰ | ��������ȫ��Ӧ�� | |
| A�� | ϴ��ƿ��ʯ��ˮ������Ϊ190 g | ϴ��ƿ��ʯ��ˮ������Ϊ196 g |
| B�� | �����ܺ���������Ʒ������Ϊ57.9 g | �����ܺ���������Ʒ������Ϊ55.2 g |
����ΪӦѡ��________���������������Ʒ������������������������Ϊ______________��
������(1)��ͼ�е�ʵ�������Ǻ�ɫ��ĩ��ڣ������ʯ��ˮ����ǣ�
(2)CO�ж�������Ⱦ��������ͼ��ȱ��β������װ�ã�
(3)��¯����ԭ���н�̿���������ṩ�������ṩ��ԭ��CO��
(4)�����Ļ�ѧԭ�������ڸ��������£��û�ԭ��һ����̼�������������е�����ԭ������ �û�ѧ����ʽ3CO��Fe2O3
�û�ѧ����ʽ3CO��Fe2O3 2Fe��3CO2��ʾ��
2Fe��3CO2��ʾ��
(5)������ ������ˮ��ʯ��ˮ���ն�����̼��һ�����ף���ˣ�����A�����ݻ�����ʵ����������ѡ��B�����ݣ����������غ㶨�ɣ������ܺ���������Ʒ���ٵ���������Ԫ�ص�����������Ԫ�ص�����Ϊ57.9 g��55.2 g��2.7 g����10 g��������Ʒ�к�������������Ϊ2.7 g��
������ˮ��ʯ��ˮ���ն�����̼��һ�����ף���ˣ�����A�����ݻ�����ʵ����������ѡ��B�����ݣ����������غ㶨�ɣ������ܺ���������Ʒ���ٵ���������Ԫ�ص�����������Ԫ�ص�����Ϊ57.9 g��55.2 g��2.7 g����10 g��������Ʒ�к�������������Ϊ2.7 g�� ��9 g������������Ʒ������������������Ϊ
��9 g������������Ʒ������������������Ϊ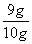 ��100%��90%��
��100%��90%��
�𰸣�(1)��ɫ��ĩ��ڣ�����ʯ��ˮ�����
(2)û��β������װ��
(3)�ṩ�������ṩ��ԭ��CO
(4)3CO��Fe2O3 2Fe��3CO2
2Fe��3CO2
(5)B��90%